Interesting Science Videos
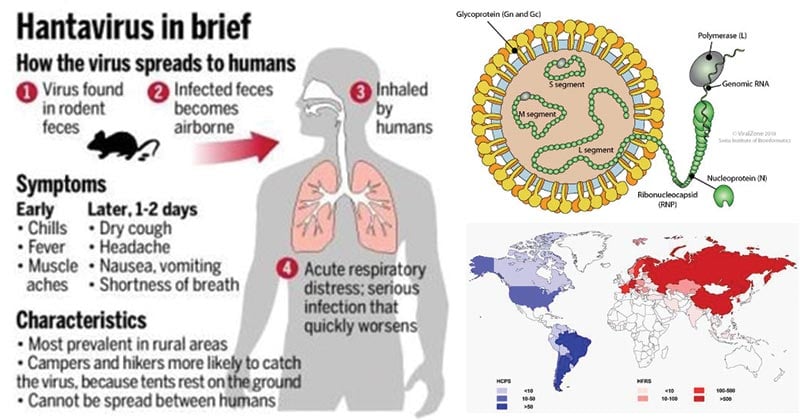
Structure of Hanta Virus
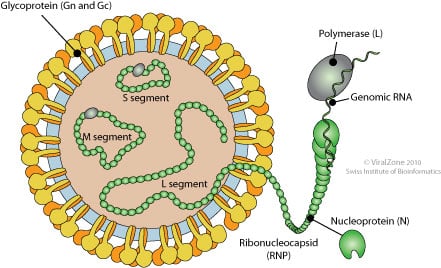
Source: Viral Zone
- Hantavirus falls under the Bunyaviridae family.
- The virion displays round or pleomorphic morphology with a diameter of roughly 120-160 nm.
- The virus capsid is enveloped by a single layer envelope.
- The envelope surrounds three nucleocapsids and has surface projections.
- Surface projections are distinctive spikes which are surrounded by a prominent fringe embedded in a lipid bilayer that is 5 nm thick.
- These projections are 5-10 nm long and they produce a grid-like structure.
- The capsid or nucleocapsid is elongated with helical symmetry.
- The ribonucleocapsid is filamentous and 200-300nm long, depending on the arrangement, and 2-2.5 nm wide.
The genome of Hanta Virus
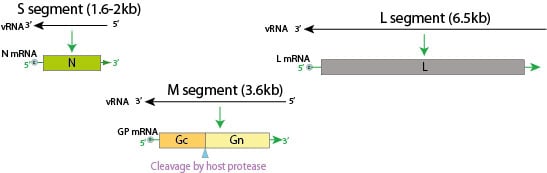
Source: Viral Zone
- The single-stranded, negative-sense, RNA genome of hantavirus is trisegmented into small, medium and large (S, M, and L) segments.
- L segment is between 6.8 and 12 kb, M segment between 3.2 and 4.9 kb and S segment between 1 and 3 kb.
- The segments, respectively, encode three structural proteins: nucleocapsid (N) protein, two glycoproteins Gn and Gc and an RNA-dependent RNA-polymerase.
- Some hantaviruses also encode a NSs protein on their S segment.
- The genome segments, encapsidated by the N protein to form ribonucleoproteins, are enclosed inside a lipid envelope that is decorated by spikes composed of Gn and Gc.
Epidemiology and Transmission of Hanta Virus
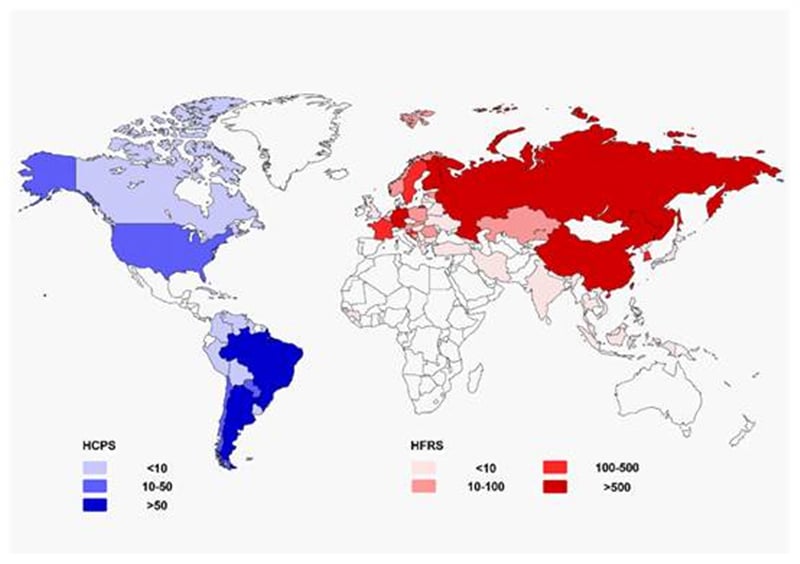
Figure: Geographical representation of approximate incidence of hantavirus cardiopulmonary syndrome (HCPS) and hemorrhagic fever with renal syndrome (HFRS) by country per year (data update to 2016). Source: https://doi.org/10.1007/s12250-016-3899-x
- Hantavirus was first identified in an outbreak in 1993 in the “Four Corners” area of the southwestern U.S. and found to be transmitted to humans by rodent urine, feces, saliva, and by airborne particles containing these items.
- The 2012 outbreak at Yosemite National Park was due to hantavirus transfer to humans by deer mice.
- Hantavirus are carried by rodents and can cause severe respiratory infections termed hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS).
- HPS is found mainly in the Americas (Canada, U.S., Argentina, Brazil, Chile, Panama, and others) while HFRS is found mainly in Russia, China, and Korea but may be found in Scandinavia and Western Europe and occasionally in other areas.
- Like HPS, HFRS results from hantaviruses that are transmitted by rodent urine, droppings, or saliva (rodent bite), by direct contact with the animals, or by aerosolized dust contaminated with rodent urine or feces to human skin breaks or to mucous membranes of the mouth, nose, or eyes.
- The vast majority of HPS and HFRS infections are not transferred from person to person.
Replication of Hanta Virus
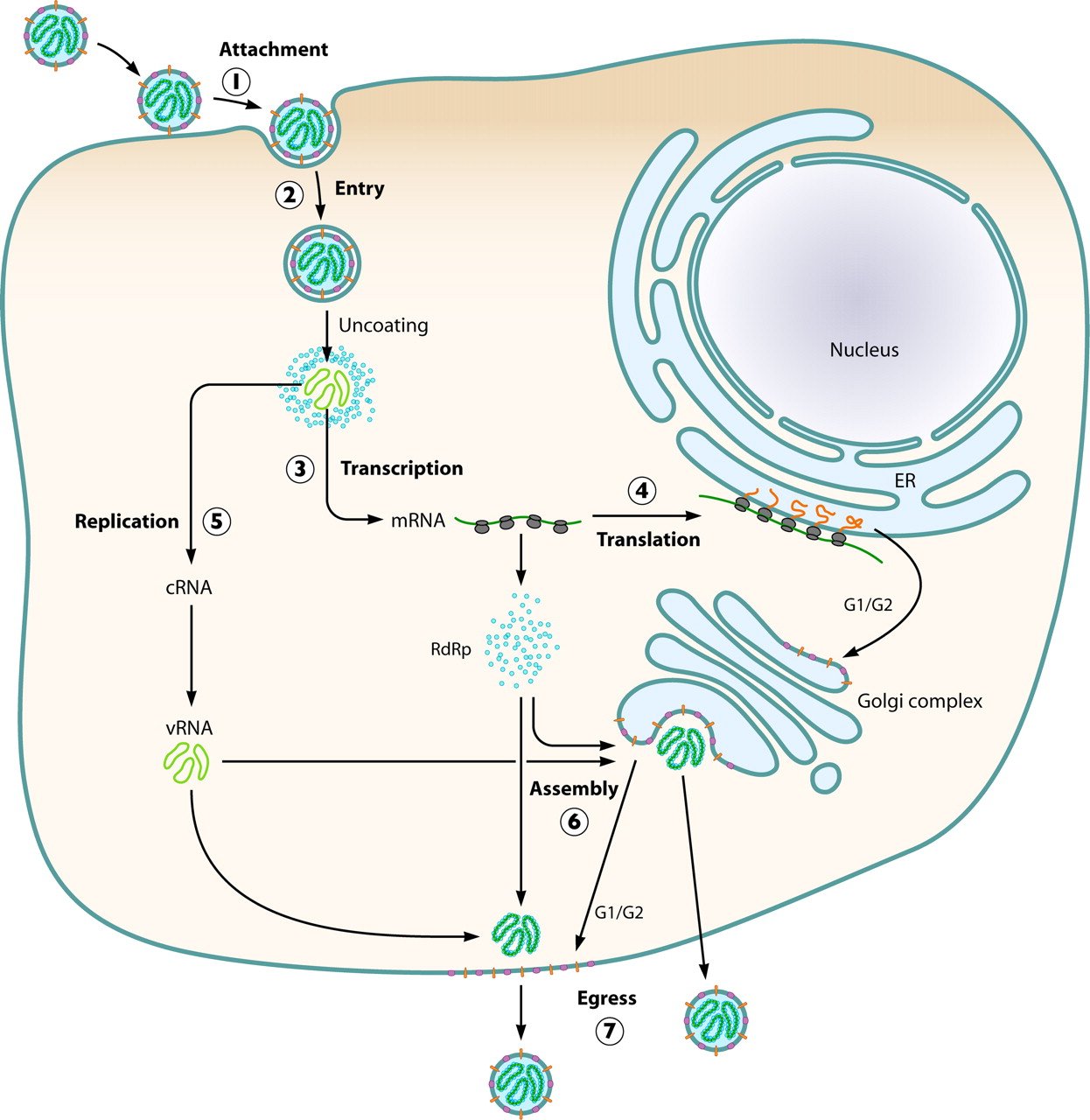
Source: DOI: 10.1128/CMR.00062-09
- The virus attaches to host receptors though Gn-Gc glycoprotein dimer, and is endocytosed into vesicles in the host cell.
- Endocytosis can occur via clathrin-mediated endocytosis, caveolin-mediated endocytosis or clathrin- and caveolin-independent endocytosis.
- The fusion of virus membrane with the vesicle membrane results in the release of ribonucleocapsid segments in the cytoplasm.
- The RNA dependent RNA polymerase (RdRp) complex initiates transcription by binding to the leader sequence in 3′ of the genomic negative-strand RNA.
- Viral mRNAs are capped in the cytoplasm.
- Replication presumably starts when enough nucleoprotein is present to encapsidate neo-synthetized antigenomes and genomes.
- The ribonucleocapsids buds at the Golgi apparatus, releasing the virion by exocytosis.
Pathogenesis of Hanta Virus
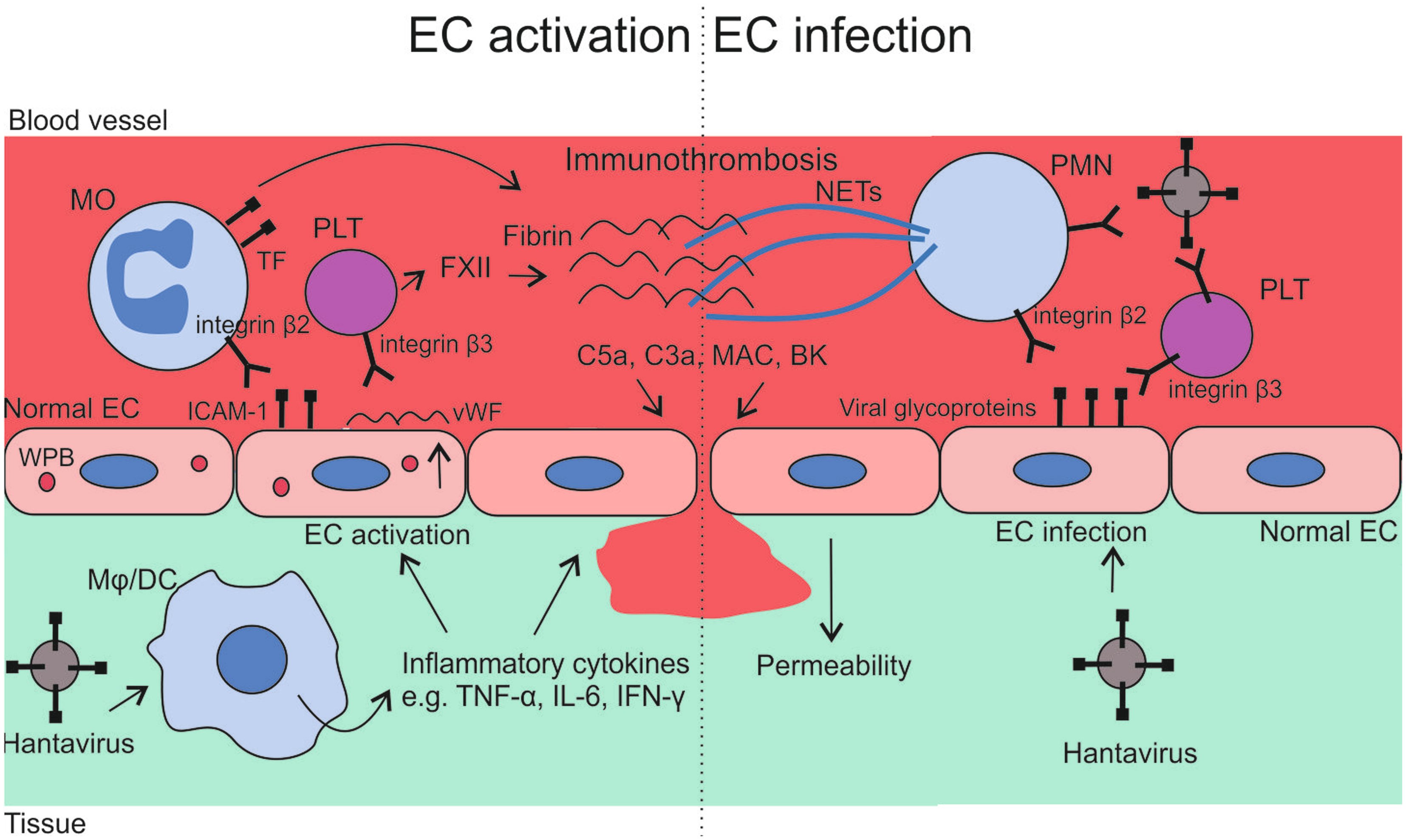
Source: https://doi.org/10.3389/fmicb.2014.00727
- Humans are infected by hantavirus mainly through inhalation of aerosolized virus-contaminated rodent excreta and the higher the number of infected rodents, the higher the risk of human infection.
- Hantaviruses cause two potentially lethal diseases, HPS and HFRS, and both diseases result in defects in vascular permeability and platelet function.
- Human beta 3 integrins confer cellular susceptibility to HPS- and HFRS-causing hantaviruses, a fact directly linking platelets, endothelial cells, and hantavirus diseases to the use of cellular receptors that maintain capillary integrity and regulate platelet function.
- The incubation period of hantavirus is relatively long: 2‒4 weeks.
- The very first barrier that the virus encounters when entering the body is the mucus gel that covers respiratory epithelial cells.
- In the lower respiratory tract, trapped microbes are removed by the ciliated cells that continually move the mucus with their cilia―away from the lungs to the throat.
- When the virus has passed the mucus gel, it encounters yet another barrier―the respiratory epithelium which has tight junction integrity and forms a particle-impermeable barrier and affects club cells.
- Despite being infected, these cells show no cytopathic effect (CPE) and the epithelial tight junction integrity is intact.
- The virus is able to infect via both the apical membrane and the basolateral membrane, and subsequently, virus particles are secreted bidirectionally.
- Once the respiratory epithelium has been traversed, the virus spreads to the lung endothelium and more distant locations in the body.
- Hantaviruses causing HFRS have tropism for cells in the kidneys, and they are found in podocytes in the glomeruli and in tubular epithelial cells of infected patients.
- In HCPS(Hantavirus Cardio Pulmonary Syndrome) patients, the virus can also be found in tubular epithelial cells but usually to a low degree.
- Studies on biopsy specimens showed inflammatory cellular infiltration close to infected lung endothelial cells in HCPS patients and close to infected kidney cells in HFRS patients.
- The infiltrations of inflammatory cells in the kidneys of HFRS patients included lymphocytes (mainly CD8+ T-cells), monocytes, macrophages, and polymorphonuclear leukocytes (including eosinophilic granulocytes and neutrophils).
- Kidney biopsy samples from HFRS patients have shown increased expression of cytokines (TNF-α, TGF-β, and PDGF) in the peritubular areas.
- Elevated serum levels of TNF-α is observed in both HCPS patients and HFRS patients.
- TNF-α is a pro-inflammatory cytokine that causes increased vascular permeability.
- Very high serum levels of IL-6 is found in fatal cases of HCPS.
- Elevated levels of IFN-γ, IL-12, and TNF-β in HCPS patients indicate an elevated Th1 response.
- The Th1 response plays a key role in antiviral immunity, but it can also produce an immunopathogenic effect.
- Infection of endothelial cells induces the production of chemokines, which attract immune cells.
- These immune cells, especially CD8+ T-cells, are thought to release an excess of cytokines (a so-called cytokine storm), which increases endothelial permeability.
Clinical Manifestations of Hanta Virus
- Hanta Virus Pulmonary Syndrome (HPS)
- Early symptoms of HPS include
- Fatigue
- Fever
- Muscle aches, especially in the thighs, hips, and back
- Headaches
- Chills
- Dizziness
- Nausea, vomiting, diarrhea or abdominal pain.
- In the late stage of infection with a hantavirus subtype, patients experience lung congestion, fluid accumulation in the lungs, and shortness of breath.
- Hemorrhagic Fever with Renal Syndrome (HFRS)
- Symptoms of HFRS usually develop within 1 to 2 weeks after exposure to infectious agents, but in rare cases, they may take up to 8 weeks to develop.
- Initial symptoms begin suddenly and include intense headaches, back and abdominal pain, fever, chills, nausea, and blurred vision.
- Individuals may have flushing of the face, inflammation or redness of the eyes, or a rash.
- Later symptoms can include low blood pressure, acute shock, vascular leakage, and acute kidney failure, which can cause severe fluid overload.
- The severity of the disease varies depending upon the virus causing the infection.
Laboratory Diagnosis of Hanta Virus
- Hantavirus isolation
- The virus can be isolated in cell culture, mostly in Vero E6 cells (green monkey kidney cells) that can only be performed by trained personnel in biosafety level 3.
- Hantavirus isolation has been carried out from fragments of organs, such as lung and liver of infected rodents and, less commonly, from human samples.
- Lacking cell-culture isolates, hantavirus is detected and characterized only by molecular methods.
- Serological diagnosis
- The detection of antihantavirus IgM and/or IgG antibodies in serum is the most common approach used for laboratory diagnosis of HCPS and HFRS.
- Diagnosis is done by indirect immunofluorescence assay (IFA) using hantavirus-infected cells fixed as an antigen in glass plates.
- Most of the hantavirus antigens used in serologic tests have been obtained by using rDNA methods.
- These antigens are mostly N-recombinant proteins (rNs), however, Gn and Gc proteins have also been produced.
- All three hantavirus structural proteins (Gn, Gc and N) can induce a high level of IgM detectable at the onset of symptoms in the infected patient.
- N, the most abundant viral protein, induces a strong humoral immune response in humans and rodents and hence, it is suitable for use as antigen in enzyme immunoassays (EIAs) for the diagnosis of hantavirus infection.
- Immunoblot and neutralization tests have also been used for the serologic diagnosis of hantavirus infections.
- Molecular diagnosis
- Highly sensitive diagnostic tests have been developed based on the detection of the virus genome.
- The hantavirus genome can be rapidly detected by reverse transcription (RT)-PCR in clinical samples, such as blood, serum or organ fragments, following on from the first day after the onset of illness.
- The real-time modality of RT-PCR is a rapid and sensitive tool for the laboratory diagnosis of hantavirus infection which allows determination of the virus load in clinical samples.
- High viral loads are indicative of severe forms of HCPS or HFRS.
- At present, the combination of a serologic test, such as the IgM ELISA with the RT-PCR, is a highly sensitive and desirable approach for the laboratory diagnosis of hantavirus infection.
Treatment
- Supportive therapy is the mainstay of care for patients with hantavirus infections.
- Care includes careful management of the patient’s fluid (hydration) and electrolyte (e.g., sodium, potassium, chloride) levels, maintenance of correct oxygen and blood pressure levels, and appropriate treatment of any secondary infections.
- Dialysis may be required to correct severe fluid overload.
- Intravenous ribavirin, an antiviral drug, has been shown to decrease illness and death associated with HFRS if used very early in the disease.
Prevention and Control
- Eliminating or minimizing contact with rodents in the home, workplace, or campsite.
- Sealing up holes and gaps in-home or garage and placing traps in and around the home to decrease rodent infestation.
- Personal hygiene and sanitation is another control measure for hantavirus infection.
References and Sources
- 6% – https://www.medscape.com/viewarticle/708397_2
- 6% – https://diseasesdic.com/hantavirus-pulmonary-syndrome/
- 4% – https://www.ncbi.nlm.nih.gov/pubmed/11217408
- 4% – https://www.medicinenet.com/pdfguides/booklet/medicinenet-daily0912.pdf
- 2% – https://www.symptoma.com/en/info/hemorrhagic-fever-with-renal-syndrome
- 2% – https://www.researchgate.net/publication/12114769_Replication_of_Hantaviruses
- 2% – https://health.mo.gov/living/healthcondiseases/communicable/communicabledisease/cdmanual/pdf/Hantavirus.pdf
- 2% – https://en.wikipedia.org/wiki/Korean_hemorrhagic_fever
- 2% – https://elbiruniblogspotcom.blogspot.com/2011/09/cdc-hemorrhagic-fever-with-renal.html
- 2% – http://health.mo.gov/living/healthcondiseases/communicable/communicabledisease/cdmanual/pdf/Hantavirus.pdf
- 1% – https://www.researchgate.net/publication/259876829_First_Molecular_Evidence_for_Puumala_Hantavirus_in_Poland
- 1% – https://www.medscape.com/viewarticle/708397_4
- 1% – https://www.medscape.com/viewarticle/708397_3
- 1% – https://www.medscape.com/viewarticle/708397
- 1% – https://www.medicinenet.com/hantavirus_pulmonary_syndrome/article.htm
- 1% – https://www.cdc.gov/hantavirus/hps/prevention.html
- 1% – https://www.cdc.gov/hantavirus/hfrs/index.html
- 1% – https://viralzone.expasy.org/by_species/213
- 1% – https://viralzone.expasy.org/7099
- 1% – https://viralzone.expasy.org/4916?outline=all_by_species
- 1% – https://viralzone.expasy.org/213
- 1% – https://bestpractice.bmj.com/topics/en-us/928/diagnosis-approach
- 1% – http://ipm.ifas.ufl.edu/pdfs/Johnson_Viruses_and_Viroids_Important_Plant_Pathogens_pics96pdi.pptx
- <1% – https://www.sciencedirect.com/topics/medicine-and-dentistry/hantavirus
- <1% – https://www.sciencedirect.com/topics/medicine-and-dentistry/inflammatory-cell
- <1% – https://www.sciencedirect.com/book/9780128031094/viruses
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4373389/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC416501/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3783221/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2715838/
- <1% – https://www.intechopen.com/books/immune-response-activation-and-immunomodulation/cytokine-profiling-plays-a-crucial-role-in-activating-immune-system-to-clear-infectious-pathogens
- <1% – https://www.healthlinkbc.ca/health-topics/hw191131
- <1% – http://www.jbc.org/content/280/6/4429.long
