Fasciola hepatica is also known as a common liver fluke or sheep liver fluke. It causes hepatic fibrosis in ruminants and humans known as Fascioliasis.
Interesting Science Videos
History and distribution of Fasciola hepatica
- Fasciola hepatica was the first fluke or trematode that was discovered more than 600 years ago in 1379 by Jehan de Brie.
- It was named by Linnaeus in 1758.
- It is the largest and most common liver fluke found in man, however, its primary host is the sheep, and to a less extent, cattle.
- It causes the economically important disease, “liver rot‘; in sheep.
- It is found in more than 70 countries, all continents except Antarctica emerging as a significant human parasitic disease.
- Its prevalence is highest in areas where extensive sheep and cattle raising occurs and where people consume raw aquatic vegetables contaminated with immature parasite larvae.
- It has been estimated that up to 17 million people are infected and that another 180 million are at risk.
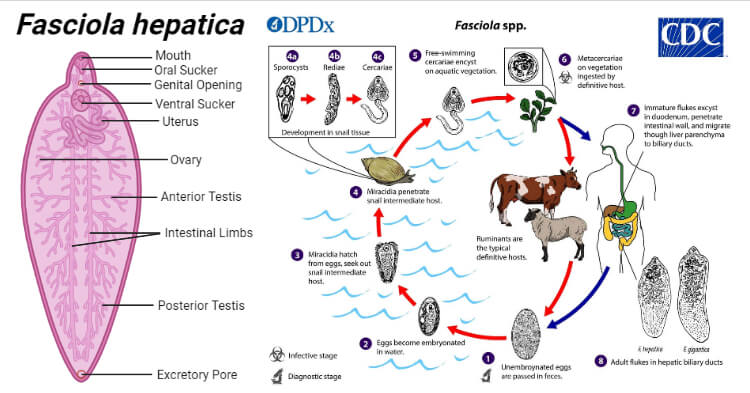
Habitat of Fasciola hepatica
It is endoparasite and lives in the liver and bile duct of the definitive host.
Morphology of Fasciola hepatica
Adult worm of Fasciola hepatica
- It is large enough to be visible to the naked eye measuring (3cm length by 1.2cm breadth), flat leaf-shaped flukes, gray or brown in color.
- It has a conical projection anteriorly containing an oral sucker and a ventral sucker at the base of the cone which allows it to attach to the lining of the biliary ducts.
- Its intestine is bifurcated and incomplete and bears lateral branches.
- The adult worm lives in the biliary tract of the definitive host for many years-about 5 years in sheep and 10 years in humans.
- Like all other trematodes, it is hermaphrodite with both male and female reproductive organs.
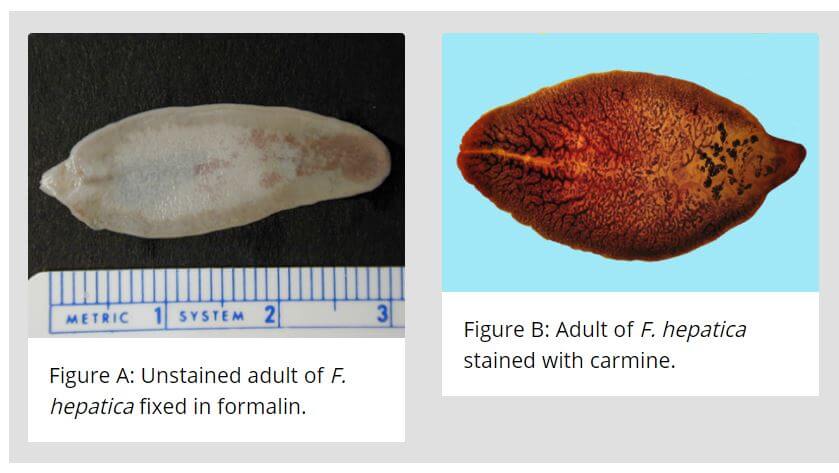
Image Source: CDC.
Eggs of Fasciola hepatica
- The eggs are yellow-brown, large, ovoid, operculated, bile-stained, and measure 140 μm by 80 μm.
- The eggshell is smooth and fined with a double line.
- Eggs contain an immature larva, the miracidium.
- F. hepatica and Fasciolopsis buski eggs cannot be differentiated.
- It unembryonated when freshly passed.
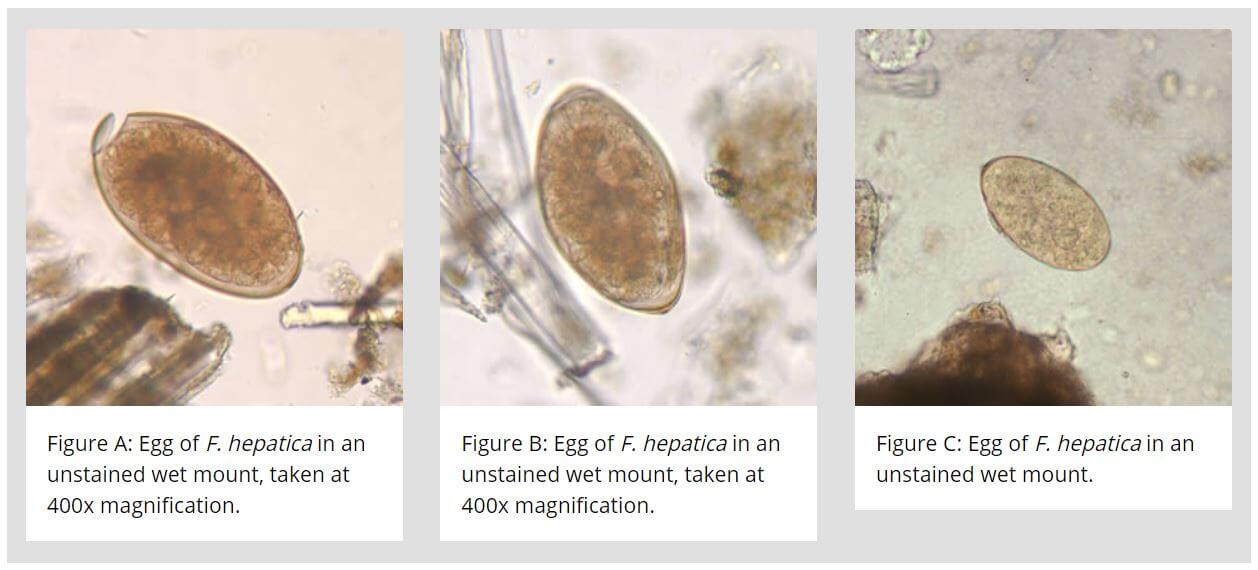
Image Source: CDC.
Larva of Fasciola hepatica
- Metacercaria larva is the infective form for man and other definitive hosts.
- Other larval forms are miracidia, rediae, and sporocysts.
Life cycle of Fasciola hepatica
- The life cycle of F. hepatica is complex and completed in two hosts.
- The primary host is sheep, goat, cattle, and man and while the intermediate host is a snail (Lymnaea, Planorbis, etc.,)
- This type of life cycle, involving two different kinds of hosts, is called digenetic.
Mode of transmission
- The sheep and other definitive hosts including man get the infection by eating water plants and watercress
containing metacercariae.
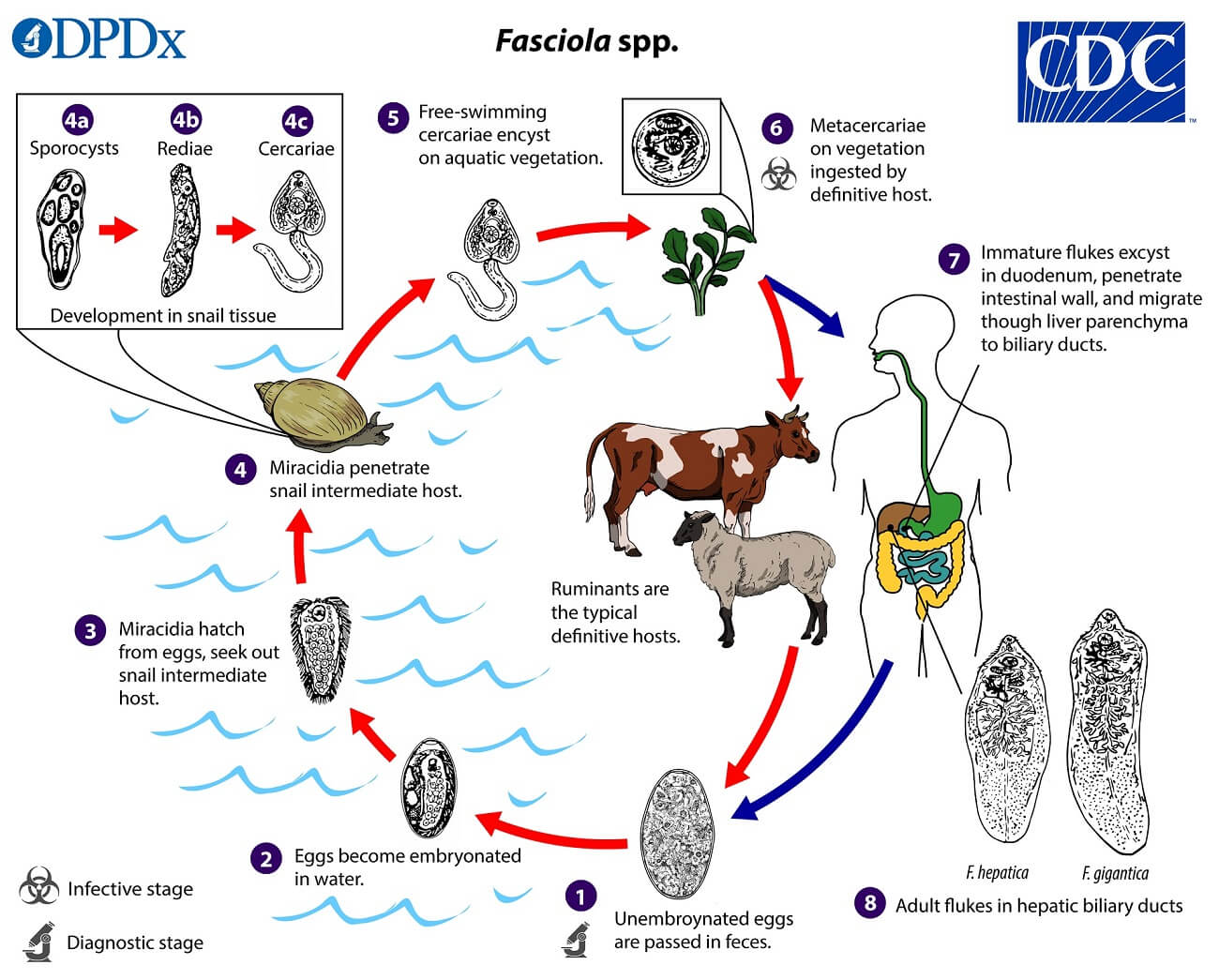
Image Source: CDC.
Development in Man or Sheep
- In the duodenum, the metacercariae excyst and the larvae emerge.
- The larvae travel through the lumen of the bowel, penetrate into the peritoneum, and invade the liver capsule. At this point, the larvae mature into adult worms in about 9 weeks of infection. and then they gradually migrate into the bile duct and bile passages.
- After several weeks in the bile duct, they become sexually mature adults and start layings unembryonated eggs which come back to the intestine and are excreted in the feces.
Development in Water
- Eggs become embryonated in freshwater over ~2 weeks to release miracidium at 22–26°C.
Development in snails
- The miracidium infects the suitable snail in particular those of the family Lymnaeidae, which are the intermediate host.
- Inside the snail, the miracidium multiplies and transforms into sporocysts, which further develop into two generations of rediae. Finally, the rediae give rise to cercariae.
Development in aquatic plants
- The cercariae escape from the snails in water and infect the water plants where they encyst to form metacercariae.
- The vegetation or water contaminated with metacercariae larvae when ingested by the definitive host causes infection and the cycle is repeated.
Clinical presentation of Fascioliasis (Fasciola hepatica)
- The incubation period varies from days to few months.
- The acute phase of infection is rarely seen in humans and occurs when a large number of metacercariae are ingested at once.
- Diseases develop during metacercarial migration (1–2 weeks after infection) and include fever, right upper quadrant pain, hepatomegaly, and eosinophilia are the most frequent symptoms although vomiting, diarrhea, urticaria (hives), anemia, and may all be present.
- The adult worm can cause obstruction of the bile duct and dilatation of the biliary tract.
- The acute phase continues for 6-8 weeks until the larvae mature and settle in the bile ducts.
- In the chronic phase, the liver parenchyma is inflamed with the formation of multiple subcapsular abscesses (called liver rot).
- Bile duct obstruction by adult worm and biliary cirrhosis are also reported but less commonly.No association to liver malignancy has been described as Fascioliasis.
- Inflammation of the bile ducts eventually leads to fibrosis and a condition called “pipestem liver“, a term describing the white appearance of the biliary ducts after fibrosis.
- The final outcome of severe infections is portal cirrhosis and even death.
- Cases of invasion into inguinal lymph nodes have been described. Other ectopic sites include subcutaneous skin, brain, and eyes.
Diagnosis of Fascioliasis (Fasciola hepatica)
Samples; stool, duodenal aspirates, or biliary aspirates
a. Stool microscopy
- Demonstration of typical operculated eggs in feces or aspirated bile from the duodenum is the best method of diagnosis.
- In the case of acute conditions, stool microscopy is not useful as the worm burden is less.
- Concentration techniques (sedimentation methods) can be followed to increase the sensitivity. Floatation methods are not useful
- The operculated eggs of F. hepatica are similar to that of F. gigantica, F. buski, Echinostoma, and Gastrodiscoides.
b. Blood picture
- Reveals eosinophilia.
c. Serological test
- In the case of ectopic infections where eggs are not present in the stool, serological tests can be used, the FAST-ELISA being most popular for the detection of specific antibodies.
- ELISA becomes positive within 2 weeks of infection and is negative after treatment. It has a sensitivity of 95%.
- Others include counter electrophoresis and the western blot technique.
- In chronic fascioliasis, Fasciola coproantigen may be detected in stool.
- They are useful for seroepidemiological study and to monitor the response to treatment.
d. Molecular Methods
- DNA probes and polymerase chain reaction (PCR) are available to detect F. hepatica-specific genes in stool specimens.
e. Imaging
- Ultrasonography, computed tomography (CT)scan or magnetic resonance imaging (MRI) can be used to detect the lesion in the liver.
- Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous cholangiography may be helpful in the diagnosis
f. Others
- Elevated serum IgE and IgG4 antibodies.
Treatment of Fascioliasis
- Currently, triclabendazole is the drug of choice selected by the Center for Disease Control and Prevention. The recommended dose is 10 mg/kg as a single dose.
- Triclabendazole is a good drug to target both immature and mature forms of the trematode, may therefore be employed during the acute and chronic phases of the treatment.
- The alternative drug is bithionol (30–50 mg for 10–15 days), Prednisolone at a dose of 10–20 mg/kg is used to control toxemia.
Prophylaxis of Fascioliasis
Fascioliasis can be prevented by following ways:
- Health education
- Improving sanitation
- Preventing pollution of watercourses with sheep, cattle, and human feces.
- Proper disinfection of watercresses and other water vegetations before consumption.
- Control of snails
- Treatment of infected person.
References
- Stephen Gillespie (Editor) and Richard D. Pearson (Editor). 2001. Principles and Practice of Clinical Parasitology. Wiley.
- Abhay R. Satoskar. 2009. Medical Parasitology. 1st edition. CRC Press.
- Sougata Ghosh. 2017. Paniker’s Textbook of Medical Parasitology. 8th edition. Jaypee Brothers Medical Pub.
Sources
- https://web.stanford.edu/group/parasites/ParaSites2001/fascioliasis/Fasciola.htm – 17%
- https://en.wikipedia.org/wiki/Fasciola_hepatica – 6%
- https://www.who.int/foodborne_trematode_infections/fascioliasis/fascioliasis_diagnosis/en/ – 2%
- https://www.coursehero.com/file/68414298/15-Parasitology-Intestinal-Lung-Flukespdf/ – 1%
- https://www.cdc.gov/parasites/fasciola/epi.html – 1%
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2867827/ – 1%
- https://en.wikipedia.org/wiki/Schistosoma – 1%
- https://www.healthline.com/health/worms-in-humans – <1%
