Interesting Science Videos
Esbach test definition
Esbach test is one of the oldest biochemical tests used to detect urinary protein like albumin when the urine is combined with citric and picric acid. Esbach test, even though not discussed much through the history, is useful for both qualitative and quantitative determination of albumin in the urine sample. The test was discovered by Georges Hubert Esbach in 1874 as one of the earliest attempts at quantification of albumin in the urine.
Esbach test has continuously been criticized for precipitating not just albumin but all other proteins in urine like peptones, mucin, and proteoses. However, later a modified version of the test to detect just the albumin and nothing else was described by Purdy. The basis of this test is the formation of precipitation when the urine is mixed with a reagent containing picric and citric acid. The quantification of the protein can then be done by measuring the height of the deposit, which gives a measured quantity of albumin. This test, nonetheless, has its limitations. Even though it has been demonstrated to detect gross changes in proteinuria, the Esbach test is too inaccurate at low levels of proteins. However, the use of a modified version of the Esbach test is encouraged by some scientists as it is a very simple method that can be performed daily and gives instant results.
Objectives of Esbach test
- To detect the presence of proteins in a sample.
- To detect the presence of albumin and other proteins in a urine sample.
Principle of Esbach test
The test is based on the principle of precipitation of albumin in the presence of organic acids. The organic acids (citric acid and picric acid) added to the sample exist as negative ions in the solution form. Similarly, the proteins in the urine sample exist as cations when the isoelectric pH of the protein is on the acidic side. Thus, it is essential that the urine sample to be tested has to be acidic enough for the dissociation to take place. When the organic acids are added to the sample, the positively charged ions of proteins combine with the negatively charged ions of the organic acids. As a result, a salt of protein is formed with results in the formation of a precipitate. For the quantitative estimation of albumin, the precipitation formed in the tube is measured by the means of graduations present on the albuminometer tube used for the test. In order to estimate just albumin, a modified version of the Esbach test is developed. As per the modified method, urine is reacted with 10% potassium ferrocyanide, and acetic acid in a specially designed graduated conical flask. After about five minutes of centrifugation, it is said that every 0.1 ml of precipitate represents 1% albumin.
Requirements
Reagent
- Esbach’s reagent: Add 10 grams of picric acid and 20 grams of citric acid to 1000 ml distilled water and mix well. A fresh reagent is preferred for a more accurate result.
- Urine sample
Materials required
- Esbach’s albuminometer: Esbach’s albuminometer is a glass tube with markings. Two distinct markings can be seen where the mark ‘U’ indicates urine level and ‘R’ indicates the reagent level. From the base of the tube to the marking ‘U’ the tube is graduated from 0-12 gram. Each of these figures represents grams of albumin per 1000 ml of urine. These gradations help to measure the quantity of precipitate formed.
- Pipettes
Procedure of Esbach test
Qualitative test
- About 3 ml of Esbach’s reagent is taken in a test tube. To this, 2 ml of a filtered urine sample is added drop by drop.
- The tube is then observed for immediate precipitation.
Quantitative test
- The urine is filtered to remove any cells or other particles.
- If the urine is not acidic enough, few drops of 10% acetic acid are added to the urine sample.
- The urine thus prepared is added to the albuminometer tube up to the ‘U’ marking.
- The reagent is then added to the tube up to the marking ‘R’.
- The tube is then closed with a rubber plug, and the contents are mixed by gently inverting the several times.
- The tube is then left at rest in the upright position for about 24 hours, and at the end of it, the scale is read to determine the amount of the deposit.
Result and Interpretation of Esbach test
Qualitative test
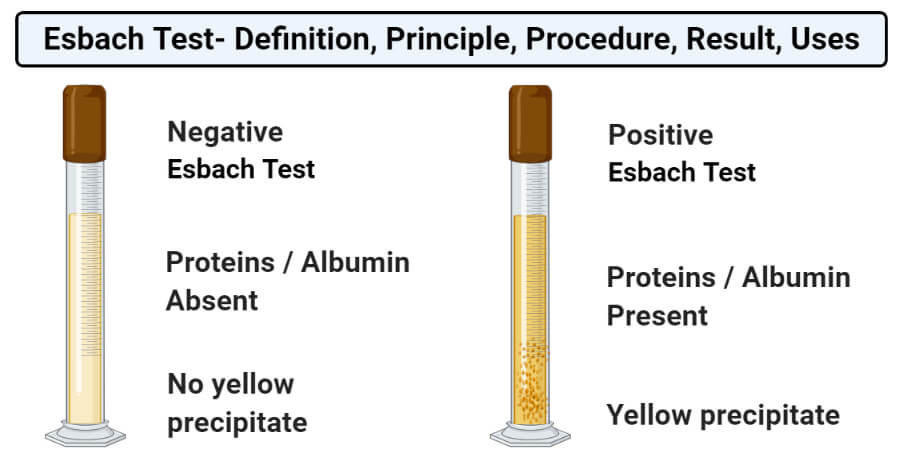
- Positive result: A positive result of the Esbach test is represented by the formation of yellow precipitate as soon as the reagent and urine sample comes in contact with one another.
- Negative result: A negative result of the Esbach test is represented by the absence of yellow precipitate or the formation of precipitate after some time.
Quantitative test
- Results of the quantitative test can be read directly. If the precipitate is level with the 4 graduations on the tube, this indicates that the urine contains 4 grams of albumin per 1000 ml urine sample.
Uses of Esbach test
- Esbach test is used as a clinical test for the quantitative estimation of albumin in the urine.
- Besides, it can also be used for the detection of other proteins in urine as well as other samples.
Limitations of Esbach test
- Esbach’s method is not strictly accurate as it might precipitate other substances along with albumin.
- Only the specimen collected over a 24 hour period should be used.
- Urine with a specific gravity that exceeds 1.010 should be diluted as it might not settle properly.
- The urine should be rendered with acid in order to increase the acidity of the sample as the precipitation only occurs under acidic conditions.
- The concentration of albumin that is less than half a gram or more than 12 grams per 1000 ml urine sample cannot be detected by this method.
References
- Veale, H. “Note on Esbach’s Method for Estimating the Quantity of Albumen in Urine.” British medical journal, vol. 1,1219 (1884): 898. doi:10.1136/bmj.1.1219.898
- Hatcher W. J (1939). Albumin in Urine. The British Journal of Nursing. April 1939.
- Rosenfeld L. (1925). Four Centuries of Clinical Chemistry. Taylor and Francis. New York.
- Calvert W. J. Esbach’s albumin test complicated by kreatinin. 1907;XLIX(3):245. doi:10.1001/jama.1907.25320030049003a
- https://www.zmchdahod.org/pdf/college/Reactions_of_Protein-01-11-2018.pdf
