The Electron Transport System also called the Electron Transport Chain, is a chain of reactions that converts redox energy available from oxidation of NADH and FADH2, into proton-motive force which is used to synthesize ATP through conformational changes in the ATP synthase complex through a process called oxidative phosphorylation.
- Oxidative phosphorylation is the last step of cellular respiration.
- This stage consists of a series of electron transfer from organic compounds to oxygen while simultaneously releasing energy during the process.
- In aerobic respiration, the final electron acceptor is the molecular oxygen while in anaerobic respiration there are other acceptors like sulfate.
- This chain of reactions is important as it involves breaking down of ATP into ADP and resynthesizing it in the process to ATP, thus utilizing the limited ATPs in the body about 300 times in a day.
- The electron flow takes place in four large protein complexes that are embedded in the inner mitochondrial membrane, together called the respiratory chain or the electron-transport chain.
- This stage is crucial in energy synthesis as all oxidative steps in the degradation of carbohydrates, fats, and amino acids converge at this final stage of cellular respiration, in which the energy of oxidation drives the synthesis of ATP.
Interesting Science Videos
Electron Transport Chain Location
- As the citric acid cycle takes place in the mitochondria, the high energy electrons are also present within the mitochondria. As a result, the electron transport chain in eukaryotes also takes place in the mitochondria.
- The mitochondrion is a double-membraned organelle that consists of an outer membrane and an inner membrane that is folded into a series of ridges called cristae.
- There are two compartments in the mitochondria; the matrix and the intermembrane space.
- The outer membrane is highly permeable to ions. It contains enzymes necessary for citric acid cycles while the inner membrane is impermeable to various ions and contains uncharged molecules, electron transport chain and ATP synthesizing enzymes.
- The number of electron transport chains in the mitochondria depends on the location and function of the cell. In the liver mitochondria, there are 10, 000 sets of electron transport chains while the heart mitochondria have three times the number of electron transport chain as in the liver mitochondria.
- The intermembrane space contains enzymes like adenylate kinase, and the matrix contains ATP, ADP, AMP, NAD, NADP, and various ions like Ca2+, Mg2+, etc.
Electron Transport Chain Components/ Electron carriers
- Electrons in the chain are transferred from substrate to oxygen through a series of electron carriers.
- There are about 15 different chemical groups that accept or transfer electrons through the electron chain.
a. FMN (Flavin Mononucleotide)
- At the beginning of the electron transfer chain, the electrons from NADH are transferred to the flavin Mononucleotide (FMN) reducing it to FMNH2.
NAD + H+ + FMN → NAD + FMNH2
- The transfer of electrons is catalyzed by the action of NADH dehydrogenase.
- The electrons are further transferred to a series of iron-sulfur complexes (Fe-S) which have a higher relative affinity towards the electrons.
b. Ubiquinone (Co-enzyme-Q)
- Between the flavoproteins and cytochromes are other electron carriers termed ubiquinone (UQ).
- Ubiquinone is the only electron carrier in the respiratory chain that is not bound attached to a protein. This allows the molecule to move between the flavoproteins and the cytochromes.
- Once the electrons are transferred from FMNH2 via the Fe-S centers to the ubiquinone, it becomes UQH2 and the oxidized form of flavoprotein (FMN) is released.
FMNH2 + UQ → FMN + UQH2
c. Cytochromes
- The next electron carriers are cytochromes that are red or brown colored proteins containing a heme group that carries the electrons in a sequence from ubiquinone to the molecular oxygen.
- However, each cytochrome, like Fe-S centers, only transfers a single electron whereas other electron carriers like FMN and ubiquinone transfer two electrons.
- There are five types of cytochromes between ubiquinone and the molecular oxygen, each designated as a, b, c, and so on.
- These are named on the basis of their ability to absorb light of different wavelengths (cytochrome a absorbs the longest wavelength, b absorbs the next longest wavelength and so on).
Electron Transport Chain Equation
The electron transport chain consists of a series of oxidation-reduction reactions that lead to the release of energy. A summary of the reactions in the electron transport chain is:
NADH + 1/2O2 + H+ + ADP + Pi → NAD+ + ATP + H2O
Electron Transport Chain Complexes
A chain of four enzyme complexes is present in the electron transport chain that catalyzes the transfer of electrons through different electron carriers to the molecular oxygen.
a. Complex I (Mitochondrial complex I)
- Complex I in the electron transport chain is formed of NADH dehydrogenases and the Fe-S centers that catalyzes the transfer of two electrons from NADH to ubiquinone (UQ).
- At the same time, the complex translocates four H+ ions through the membrane, creating a proton gradient.
NADH + H+ + CoQ → NAD+ + CoQH2
- NADH is first oxidized to nAD+ by reducing FMN to FMNH2 in a two-step electron transfer.
- FMNH2 is then oxidized to FMN where the two electrons are first transferred to Fe-S centers and then to ubiquinone.
b. Complex II (Mitochondrial complex II)
- Complex II consists of succinic dehydrogenase, FAD, and Fe-S centers.
- The enzyme complex catalyzes the transfer of electrons from other donors like fatty acids and glycerol-3 phosphate to ubiquinone through FAD and Fe-S centers.
- This complex runs parallel to the Complex II, but Complex II doesn’t translocate H+ across the membrane, as in Complex I.
Succinate + FADH2 + CoQ → Fumarate + FAD+ + CoQH2
c. Complex III (Mitochondrial complex III)
- Complex III consists of cytochrome b, c, and a specific Fe-S center.
- The enzyme complex, cytochrome reductase, catalyzes the transfer of two electrons from reduced CoQH2 to two molecules of cytochrome c.
- Meanwhile, the protons (H+) from the ubiquinone are release across the membrane aiding to the proton gradient.
- The CoQH2 is oxidized back to CoQ while the iron center (Fe3+) in the cytochrome c is reduced to Fe2+.
CoQH2 + 2 cytc c (Fe3+) → CoQ + 2 cytc c (Fe2+) + 4H+
d. Complex IV (Mitochondrial complex IV)
- Complex IV consists of cytochrome a and a3, which is also termed cytochrome oxidase.
- This is the last complex of the chain and is involved in the transfer of two electrons from cytochrome c to molecular oxygen (O2) forming water.
- In the meantime, four protons are translocated across the membrane aiding the proton gradient.
4 cytc c (Fe 2+) + O2 → 4cytc c (Fe3+) + H2O
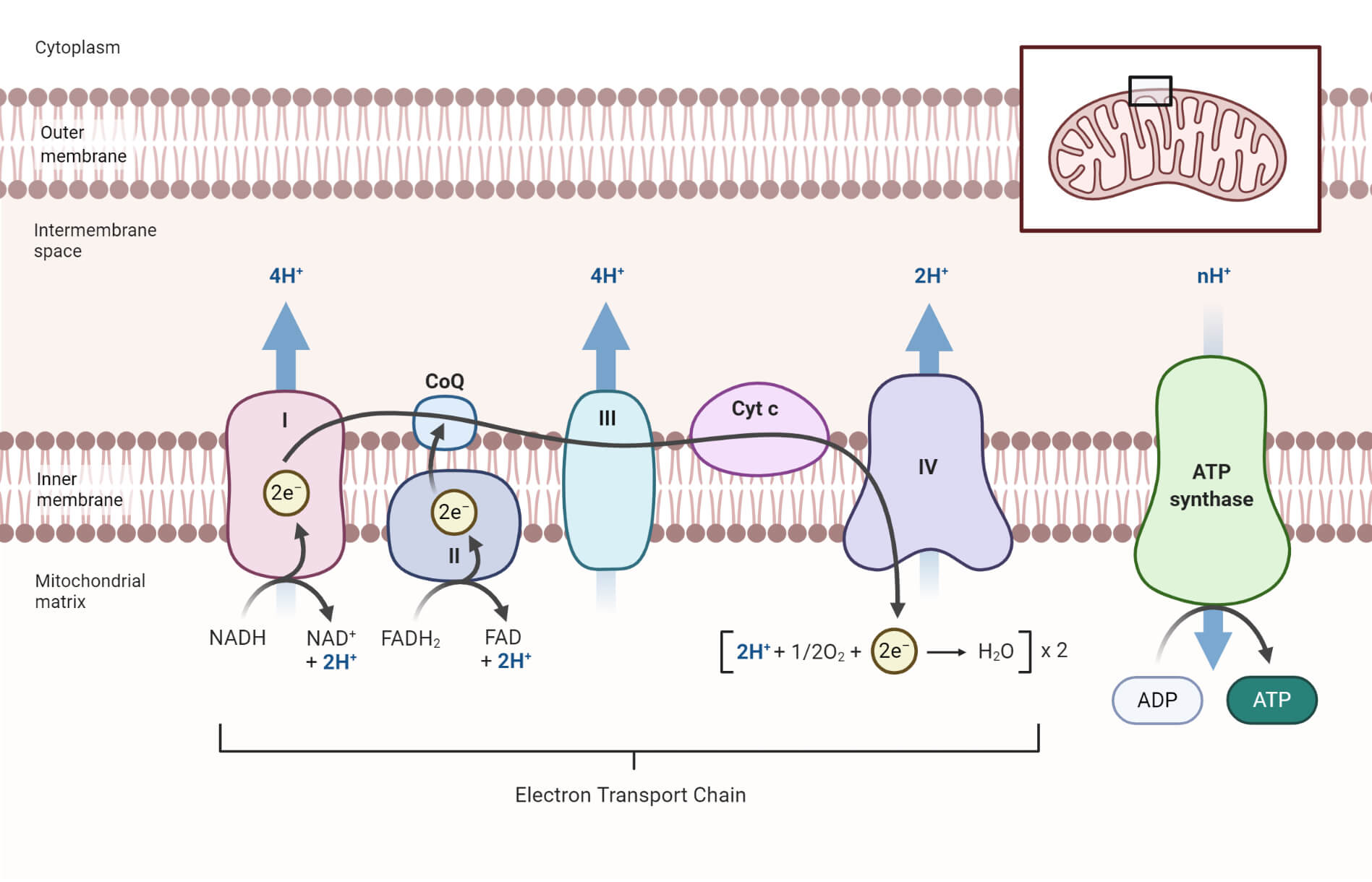
Figure: Electron Transport Chain. Image created with biorender.com
Electron Transport Chain Steps
The following steps are involved in electron transfer chains which involve the movement of electrons from NADH to molecular oxygen:
1. Transfer of electrons from NADH to Ubiquinone (UQ)
- NADH is produced in different other cycles by the α-ketoglutarate dehydrogenase, isocitrate dehydrogenase, and malate dehydrogenase reactions of the TCA cycle, by the pyruvate dehydrogenase reaction that converts pyruvate to acetyl-CoA, by β-oxidation of fatty acids, and by other oxidation reactions.
- NADH produced in the mitochondrial matrix is transferred into the intermembrane space.
- The NADH then transfers the electrons to FMN present in the intermembrane space via the complex I (NADH dehydrogenase).
- FMN then passes the electrons to the Fe-S center (one electron to one Fe-S center) which then transfers the electrons, one at a time to CoQ forming semiquinone and then ubiquinol.
- The electron transfer creates energy which is used to pump two protons across the membrane creating a potential gradient.
- The protons move back to the matrix through the pore in the ATP synthase complex, forming energy in the form of ATP.
2. Transfer of electrons from FADH2 to CoQ
- The oxidation of succinate to fumarate results in the reduction of FAD to FADH2.
- The electrons from FADH2 enter the electron transport chain catalyzed by complex II, succinic dehydrogenase.
- Like in complex I, the electrons reach CoQ through a series of Fe-S centers.
- However, complex II doesn’t pump any protons across the membrane.
3. Transfer of electrons from CoQH2 to cytochrome c
- The reduced CoQH2 transfer electrons through cytochrome b and c1 which finally reaches cytochrome c.
- Complex II (cytochrome reductase) catalyzes this process where the Fe3+ present in the cytochrome is reduced to Fe2+.
- Each cytochrome transfers one electron each and thus two molecules of cytochrome are reduced for the transfer of electrons for every NADH oxidized.
- Energy is produced during the transfer of electrons which is utilized to pump protons across the membrane aiding to the potential gradient.
- The protons move back to the matrix through the pore in the ATP synthase complex, forming energy in the form of ATP like in the first step.
4. Transfer of electrons from cytochrome c to molecular oxygen
- The final step in the electron transfer chain is catalyzed by complex IV (cytochrome oxidase) where electrons are transferred from cytochrome c to molecular oxygen.
- Since two electrons are required to reduce one molecule of oxygen to water, for each NADH oxidized half of oxygen is reduced to water.
- Similarly, the Fe2+ of the cytochrome c is oxidized to Fe3+. The energy released during this process is used to pump protons across the membrane.
- The transfer of protons back to the matrix results in the formation of ATP.
Electron Transport Chain Products
The end products of the electron transport chain are:
30-32 ATPs and 44 moles of H2O
| Stage | Direct products (net) | Ultimate ATP yield (net) |
|---|---|---|
| Glycolysis | 2 ATP | 2 ATP |
| 2 NADH | 3-5 ATP | |
| Pyruvate oxidation | 2 NADH | 5 ATP |
| Citric acid cycle | 2 ATP/GTP | 2 ATP |
| 6 NADH | 15 ATP | |
| 2 FADH2 | 3 ATP | |
| Total | 30-32 ATP |
Table Source: Khan Academy
Note: In some cases, we can see the production of 38 ATPs also.
Frequently Asked Questions (FAQs) (Revision questions and answers)
Where is the electron transport chain located?
The electron transport chain is located in the mitochondria of a cell.
What is the purpose of the electron transport chain?
The purpose of electron transfer chains is the production of ATPs.
What does the electron transport chain do?
The electron transport chain produces ATPs from the precursors (NADH and FADH) of previous cycles.
What are the three main steps in the electron transport chain?
The three main steps of the electron transfer chain are:
a. Transfer of electrons from NADH and FADH2 to CoQ
b. Transfer of electrons from CoQ to cytochrome c
c. Transfer of electrons from cytochrome c to molecular oxygen.
Where are the proteins of the electron transport chain located?
The proteins of the electron transport chain are located in the inner mitochondrial membrane of the mitochondria.
What are the products of the electron transport chain?
The products of the electron transport chains are ATPs and water.
What is the final electron acceptor of the electron transport chain?
The final electron acceptor in aerobic respiration is molecular oxygen while in anaerobic respiration, it can be sulfate or other molecules.
How many ATPs are formed in the electron transport chain?
A total of 30-32 ATPs are formed in the electron transport chain. But it depends upon the ATP per glucose in cellular respiration. In some cases, we can see the production of 38 ATPs also.
How many ATPs are utilized in the electron transport chain?
No ATPs are utilized in the electron transport chain.
What is the main function of the electron transport chain?
The main function of the electron transport chain is the production of ATPs from NADH and FADH.
What is the role of oxygen in the electron transport chain?
Oxygen in the electron transport chain is the final electron acceptor.
How does electron transport chain work in cellular respiration?
Electron transport chain is the final stage of cellular respiration where most of the ATPs or energy is produced from glucose.
References
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition.
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company.

Well tabulated. Easy understanding
Well explained with all points,easy to understand,Thank you