The Krebs cycle, also known as the citric acid cycle or TCA cycle, is a series of reactions that take place in the mitochondria, resulting in the oxidation of acetyl CoA to release carbon dioxide and hydrogen atoms that later lead to the formation of water.
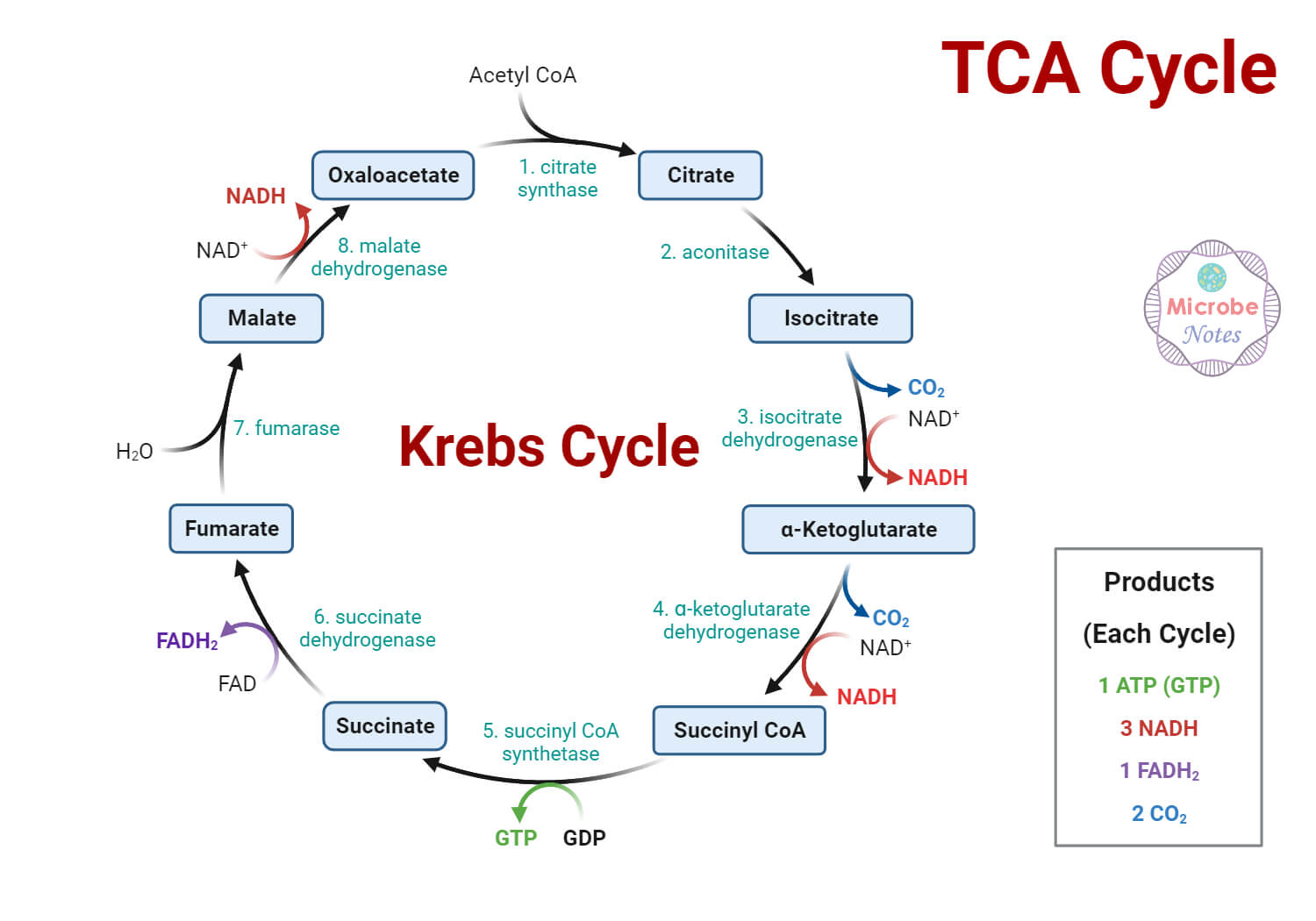
- This cycle is termed the citric acid cycle as the first metabolic intermediate formed in the cycle is citric acid.
- This cycle is also termed tricarboxylic acid (TCA) because it was then not certain whether citric acid or some other tricarboxylic acid (g., isocitric acid) was the first product of the cycle. However, now it has been known that the first product is indeed citric acid and thus the use of this name has since been discouraged.
- This cycle only occurs under aerobic conditions as energy-rich molecules like NAD+ and FAD can only be retrieved from their reduced form once they transfer electrons to molecular oxygen.
- The citric acid cycle is the final common pathway for the oxidation of all biomolecules; proteins, fatty acids, carbohydrates. Molecules from other cycles and pathways enter this cycle through Acetyl CoA.
- The citric acid cycle is a cyclic sequence of reactions formed of 8 enzyme-mediated reactions.
- This cycle is also particularly important as it provides electrons/ high-energy molecules to the electron transport chain for the production of ATPs and water.
- Pyruvate formed at the end of glycolysis is first oxidized into Acetyl CoA which then enters the citric acid cycle.
Interesting Science Videos
Krebs Cycle Location
- The citric acid cycle in eukaryotes takes place in the mitochondria while in prokaryotes, it takes place in the cytoplasm.
- The pyruvate formed in the cytoplasm (from glycolysis) is brought into the mitochondria where further reactions take place.
- The different enzymes involved in the citric acid cycle are located either in the inner membrane or in the matrix space of the mitochondria.
Krebs Cycle Equation/ Reaction
The overall reaction/ equation of the citric acid cycle is:
Acetyl CoA + 3 NAD+ + 1 FAD + 1 ADP + 1 Pi → 2 CO2 + 3 NADH + 3 H+ + 1 FADH2 + 1 ATP
In words, the equation is written as:
Acetyl CoA + Nicotinamide adenine dinucleotide + Flavin adenine dinucleotide + Adenosine diphosphate + Phosphate → Pyruvate + Water + Adenosine triphosphate + Nicotinamide adenine dinucleotide + Hydrogen ions
Krebs Cycle Enzymes
In eukaryotic cells, the enzymes that catalyze the reactions of the citric acid cycle are present in the matrix of the mitochondria except for succinate dehydrogenase and aconitase, which are present in the inner mitochondrial membrane.
One common characteristic in all the enzymes involved in the citric acid cycle is that nearly all of them require Mg2+
The following are the enzymes that catalyze different steps throughout the process of the citric acid cycle:
- Citrate synthase
- Aconitase
- Isocitrate dehydrogenase
- α-ketoglutarate dehydrogenase
- Succinyl-CoA synthetase
- Succinate dehydrogenase
- Fumarase
- Malate dehydrogenase
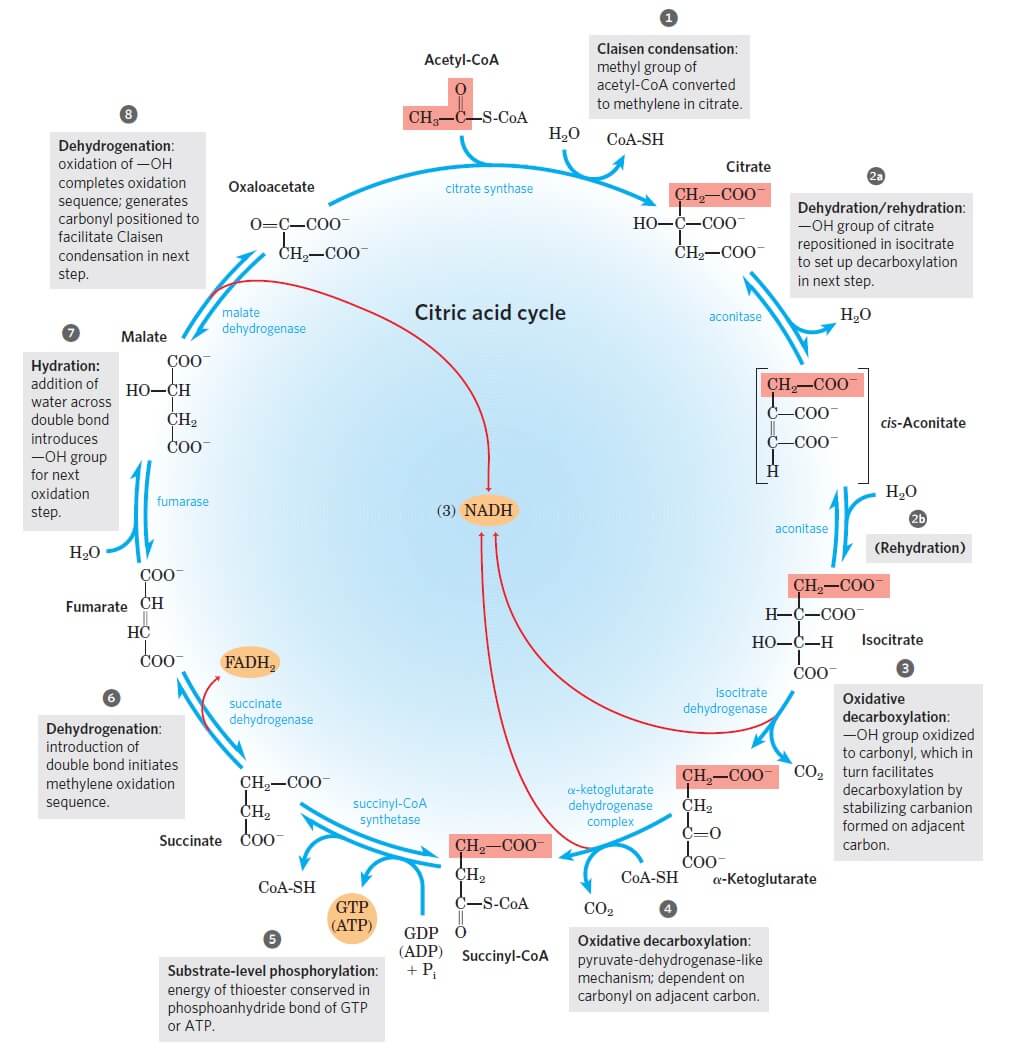
Figure: Reactions of the citric acid cycle. Image Source: Lehninger Principles of Biochemistry.
Video of Krebs Cycle
Krebs Cycle Steps
After glycolysis, in aerobic organisms, the pyruvate molecules are carboxylated to form acetyl CoA and CO2.
Oxidative Decarboxylation of pyruvate to Acetyl CoA
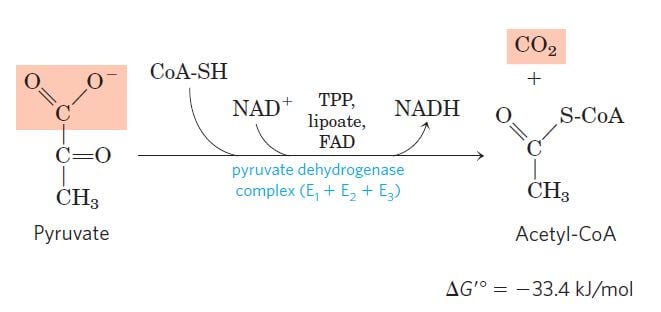
Image Source: Lehninger Principles of Biochemistry.
- The oxidative decarboxylation of pyruvate forms a link between glycolysis and the citric acid cycle.
- In this process, the pyruvate derived from glycolysis is oxidatively decarboxylated to acetyl CoA and CO2 catalyzed by the pyruvate dehydrogenase complex in the mitochondrial matrix in eukaryotes and in the cytoplasm of the prokaryotes.
- From one molecule of glucose, two molecules of pyruvate are formed, each of which forms one acetyl CoA along with one NADH by the end of the pyruvate oxidation.
- The acetyl CoA formed from pyruvate oxidation, fatty acid metabolism, and amino acid pathway then enter the citric acid cycle.
The following are the eight enzyme-catalyzed reactions/ steps in the aerobic oxidation of glucose through the citric acid cycle:
Step 1: Condensation of acetyl CoA with oxaloacetate
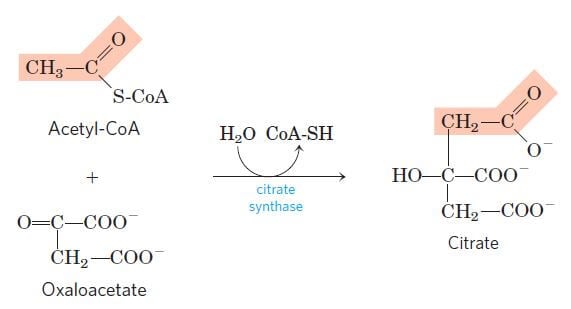
Image Source: Lehninger Principles of Biochemistry.
- The first step of the citric acid cycle is the joining of the four-carbon compound oxaloacetate (OAA) and a two-carbon compound acetyl CoA.
- The oxaloacetate reacts with the acetyl group of the acetyl CoA and water, resulting in the formation of a six-carbon compound citric acid, CoA.
- The reaction is catalyzed by the enzyme citrate synthase that condenses the methyl group of acetyl CoA and the carbonyl group of oxaloacetate resulting in citryl-CoA which is later cleaved to free coenzyme A and to form citrate.
Step 2: Isomerization of citrate into isocitrate
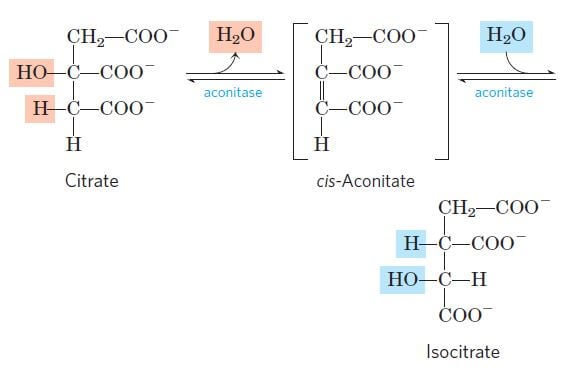
Image Source: Lehninger Principles of Biochemistry.
- Now, for further metabolism, citrate is converted into isocitrate through the formation of intermediate cis-aconitase.
- This reaction is a reversible reaction catalyzed by the enzyme aconitase.
- This reaction takes place by a two-step process where the first step involves dehydration of citrate to cis-aconitase, followed by the second step involving rehydration of cis-aconitase into isocitrate.
Step 3: Oxidative decarboxylations of isocitrate
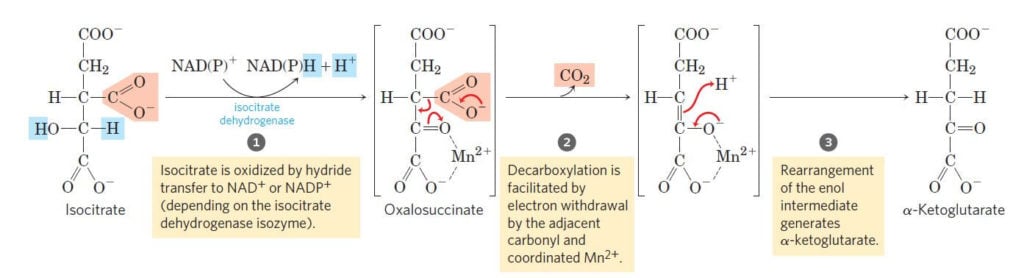
Image Source: Lehninger Principles of Biochemistry.
- The third step of the citric acid cycle is the first of the four oxidation-reduction reactions in this cycle.
- Isocitrate is oxidatively decarboxylated to form a five-carbon compound, α-ketoglutarate catalyzed by the enzyme isocitrate dehydrogenase.
- This reaction, like the second reaction, is a two-step reaction.
- In the first step, isocitrate is dehydrogenated to oxalosuccinate while the second step involves the decarboxylation of oxalosuccinate to α-ketoglutarate.
- Both the reactions are irreversible and catalyzed by the same enzyme.
- The first step, however, results in the formation of NADH while the second step involves the release of CO2.
Step 4: Oxidative decarboxylation of α-ketoglutarate
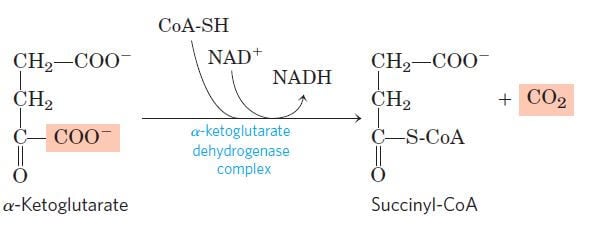
Image Source: Lehninger Principles of Biochemistry.
- This step is another one of the oxidation-reduction reactions where α-ketoglutarate is oxidatively decarboxylated to form a four-carbon compound, succinyl-CoA, and CO2.
- The reaction irreversible and catalyzed by the enzyme complex α-ketoglutarate dehydrogenase found in the mitochondrial space.
- This reaction is similar to the oxidative decarboxylation of pyruvate involving the reduction of NAD+ into NADH.
Step 5: Conversion of succinyl-CoA into succinate
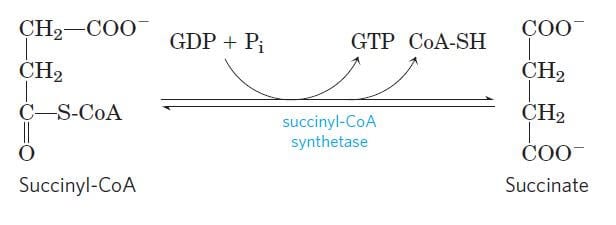
Image Source: Lehninger Principles of Biochemistry.
- In the next step, succinyl-CoA undergoes an energy-conserving reaction in which succinyl-CoA is cleaved to form succinate.
- This reaction is accompanied by phosphorylation of guanosine diphosphate (GDP) to guanosine triphosphate (GTP).
- The GTP thus formed then readily transfers its terminal phosphate group to ADP forming an ATP molecule.
- The reaction is catalyzed by the enzyme, succinyl-CoA synthase.
Step 6: Dehydration of succinate to fumarate
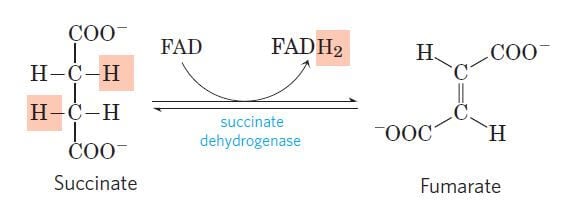
Image Source: Lehninger Principles of Biochemistry.
- Here, the succinate formed from succinyl-CoA is dehydrogenated to fumarate catalyzed by the enzyme complex succinate dehydrogenase found in the intramitochondrial space.
- This is the only dehydrogenation step in the citric acid cycle in which NAD+ doesn’t participate.
- Instead, another high-energy electron carrier, flavin adenine dinucleotide (FAD) acts as the hydrogen acceptor resulting in the formation of FADH2.
- The FADH2 then enters the electron transport chain via the complex II transferring the electrons to ubiquinone, finally forming 2ATPs.
Step 7: Hydration of fumarate to malate
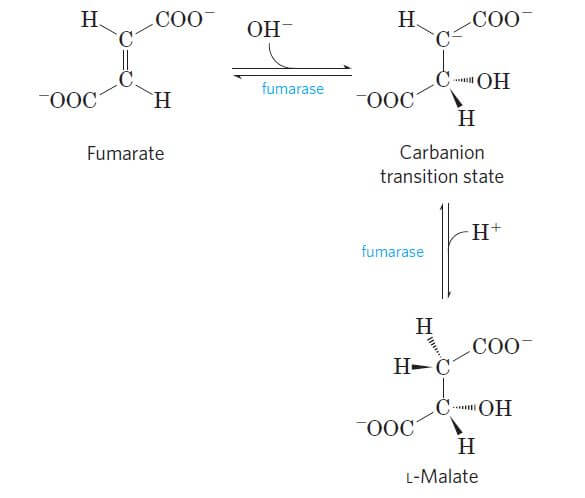
Image Source: Lehninger Principles of Biochemistry.
- The fumarate is reversibly hydrated to form L-malate in the presence of the enzyme fumarate hydratase.
- As it is a reversible reaction, the formation of L-malate involves hydration, whereas the formation of fumarate involves dehydration.
Step 8: Dehydrogenation of L-malate to oxaloacetate
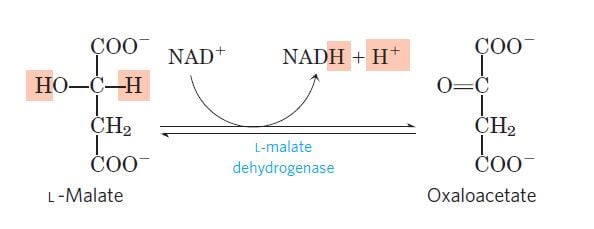
Image Source: Lehninger Principles of Biochemistry.
- The last step of the citric acid cycle is also an oxidation-reduction reaction where L-malate is dehydrogenated to oxaloacetate in the presence of L-malate dehydrogenase, which is present in the mitochondrial matrix.
- This is a reversible reaction involving oxidation of L-malate and reduction of NAD+ into NADH.
- Oxaloacetate thus formed, allows the repetition of the cycle and NADH formed participates in the oxidative phosphorylation.
- This reaction completes the cycle.
Krebs Cycle Products
Since this is a cyclic process, the oxaloacetate is formed at the end as it condenses with acetyl CoA in the next cycle.
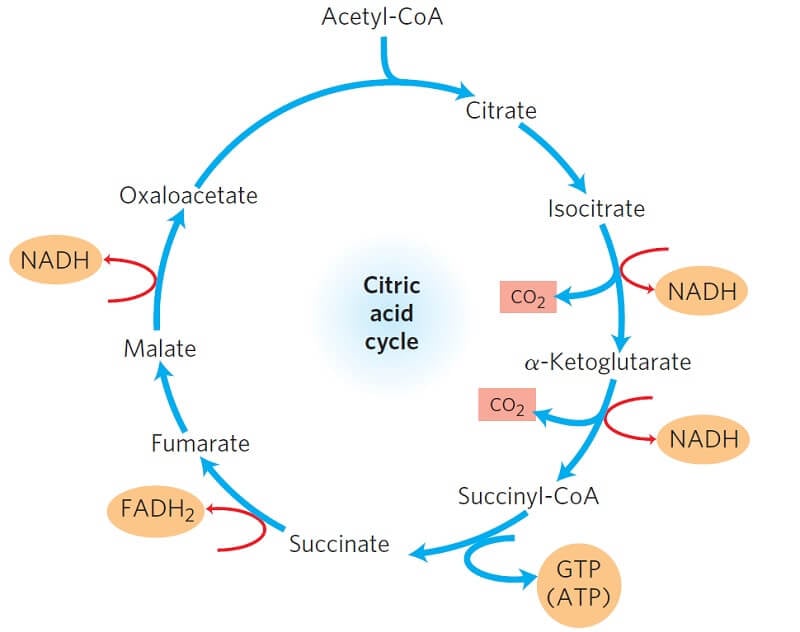
Figure: Products of one turn of the citric acid cycle. Image Source: Lehninger Principles of Biochemistry.
At each turn of the cycle,
- 3 NADH,
- 1 FADH2,
- 1 GTP (or ATP),
- 2 CO2
Note: One NADH are formed from a molecule of pyruvate in the oxidative decarboxylation of pyruvate to Acetyl CoA.
Krebs Cycle FAQs and Practice Questions
What is the purpose of the Krebs cycle?
The purpose of the Kreb’s cycle is the complete oxidation of glucose, resulting in energy-rich molecules that later produce ATPs in the electron transport chain.
Where does the Krebs cycle take place?
Krebs cycle takes place in the mitochondria of eukaryote and in the cytoplasm of the prokaryotes.
How many ATP are produced in the Krebs cycle?
One ATP is formed in a single Krebs cycle while two ATPs are formed from a single molecule of glucose (two molecules of pyruvate are formed from one molecule of glucose).
Does the Krebs cycle require oxygen?
Yes, the Krebs cycle requires oxygen as the cycle operates only under aerobic conditions as NAD+ and FAD can be regenerated from their reduced form in the mitochondria only by electron transfer to molecular oxygen.
Where do the reactions of the Krebs cycle occur in prokaryotic cells?
The reactions of the Krebs cycle occur in the cytoplasm in prokaryotic cells.
Where do the reactions of the Krebs cycle occur in eukaryotic cells?
The reactions of the Krebs cycle occur in the mitochondria in eukaryotic cells.
What inhibits the Krebs cycle?
Various factors like the absence of oxygen, low levels of oxaloacetate or pyruvate, necessary enzymes and coenzymes, high levels of ATP and NADH, and the accumulation of ketone bodies.
Krebs Cycle Song
References
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition.
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company.
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 17.2, Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22347/


Great job ✔️
But I suggest you to involve ‘Human Nutrition and Dietetics’ in your contents of discussion 🙏
This content is so comprehensive, keep up the good work.
Nicely explained step by step and elaborated way giving details about byproduct, compare to others.
Thank you
Hello!
Thank you for the detailed outline of Kreb’s cycle. I have some questions that I have tried to find the answer to. In the first figure (Figure: Reactions of the citric acid cycle.), posted just above the video it includes the water molecules. 3 water molecules are needed for the cycle and one molecule of water is produced.
Question 1: Where do the water molecules come from?
Question 2: What is the net water amount for the cycle? Is it a positive or negative number?
Not every diagram of Kreb’s cycle includes water molecules. So is it trivial?
Question 3: Where do the water molecules go?
We (Grade 12 Bio class; Erindale SS in Mississauga, Ontario, Canada) would like to know.
Thank you!
Karen Haywood (teacher)
Good thank you very much ….keep up about etc.
I found this website while researching for resources, over the Krebs Cycle.
Thank you for providing thorough explanations over this topic. I am a nursing student & your illustrations & song make this topic a bit more fun & easy to comprehend!
Hi,
We are happy to hear that you liked our website and it was helpful for you.
Thanks,
– Sagar
Excellent. Didactic. Elucidative.
Thank you so much.