Interesting Science Videos
Ehrlich Test Definition
Ehrlich Test is a biochemical test used for the detection of amino acid, tryptophan, in a protein sample. Ehrlich test is also termed the p-dimethylamino benzaldehyde test, named after the Ehrlich reagent. The test is named after the Nobel Prize winner Paul Ehrlich, who identified this test during the distinction of typhoid from simple diarrhea. It is also called a specific amino acid test as it detects a certain amino acid, tryptophan. This test has been used in laboratories for medical tests, some for drug testing while others for diagnosis of various diseases. Diagnosis of liver diseases, carcinoid syndrome, hemolytic processes, and occlusion of the common bile duct can also be performed with the Ehrich test. One of the common forms of the Ehrlich test is the spot test used for the detection of psychoactive compounds like tryptamines and ergoloids (like, LSD). A positive Ehrlich test is obtained for natural opium as it contains tryptophan. The Ehrlich reagent is also used for the identification of indoles and urobilinogen (found in urine).
Objectives of Ehrlich Test
- To detect the presence of tryptophan in a given sample.
- To distinguish between typhoid and diarrhea.
- To identify the presence of urobilinogen in a urine sample.
Principle of Ehrlich Test
Ehrlich reagent consists of p-dimethylamino benzaldehyde. The reaction occurring in the test is based on the principle that under acidic conditions, the Ehrlich’s reagent undergoes electrophilic substitution. The substitution occurs at the indole or the benzyl pyrrole ring of tryptophan to yield a blue-violet condensation product. The condensation product formed after the reaction is further enhanced by the addition of NaNO2.
Reaction
Tryptophan + Ehrlich reagent (p-dimethylamino benzaldehyde) → Blue-violet condensation product
Requirements
Reagents
- Ehrlich reagent: Ehrlich reagent is prepared by the addition of about 0.5 to 2.0 grams of p-dimethylamino benzaldehyde in 50 ml of 95% ethanol. To this, 50 ml 10% H2SO4 is added.
- Protein sample
- Concentration HCl
- NaNO2
Materials required
- Test tubes
- Test tube stand
- Pipettes
Procedure of Ehrlich Test
- About 3-4 ml of diluted protein solution or urine sample is added to a test tube.
- The liquid is then boiled with 1 ml of concentrated HCl.
- A few drops of the Ehrlich reagent (p-dimethylamino benzaldehyde in H2SO4) are added to the test tube.
- The test tube is then shaken to mix the contents together properly.
- The test tube is observed for the color change. If a blue-violet color is observed, a few drops of NaNO2 solution are added to the test tube to obtain a blue color.
Result and Interpretation of Ehrlich Test
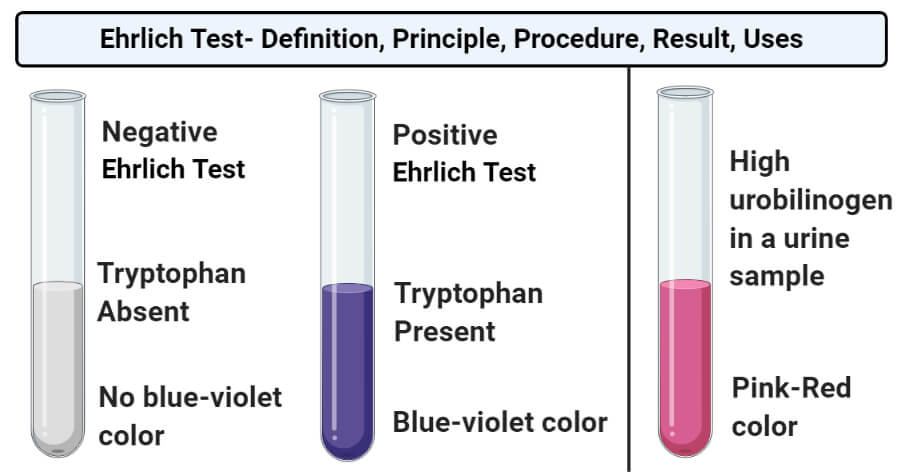
- Positive result: A positive result in the Ehrlich test is indicated by the appearance of red to purple or blue-violet color. The color then changes to blue with the addition of NaNO2. This indicates that the sample contains tryptophan.
- Negative result: A negative result in the Ehrlich test is indicated by the absence of blue-violet color on the addition of the Ehrlich reagent. This indicates that the sample doesn’t contain any tryptophan.
Uses
- This test is an aldehyde test used for the detection of tryptophan in a protein sample.
- The test is used for the diagnosis of diseases like typhoid and other disorders of the hemolytic process of the obstruction of the common bile duct.
- The test can also be used for the detection of psychoactive compounds and drugs like tryptamines and ergoloids.
- Ehrlich test detects the presence of indoles and urobilinogen. The presence of urobilinogen in high concentration in urine sample helps in the diagnosis of hepatic jaundice and hepatitis.
Limitations
- The false-negative reaction might occur in the presence of urinary tract infection as nitrites oxidize urobilinogen to urobilin.
- A false negative result during antibiotic therapy as some gut bacteria that produce urobilinogen might get destroyed.
- This test is a specific test for aldehyde or tryptophan, and it doesn’t detect other amino acids or proteins.
References and Sources
- Nigam S. C. and Omkar (2003). Experimental Animal Physiology and Biochemistry. New Age International Pvt. Limited. New Delhi.
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- WILSON, T M, and L S P DAVIDSON. “Ehrlich’s aldehyde test for urobilinogen.” British medical journal, Vol. 1, 4611 (1949): 884-7. doi:10.1136/bmj.1.4611.884
- 2% – https://medlineplus.gov/lab-tests/urobilinogen-in-urine/
- 2% – https://fac.ksu.edu.sa/sites/default/files/Qualitative_tests_of_amino_acids_Lab_1.pdf
- 1% – https://www.sigmaaldrich.com/analytical-chromatography/microbiology/microbiology-products.html?TablePage=17928001
- 1% – https://www.sciencedirect.com/topics/neuroscience/urobilinogen
- 1% – https://www.organic-chemistry.org/synthesis/heterocycles/benzo-fused/indoles.shtm
- 1% – https://nutrientsinfooddoubelc.weebly.com/food-testing-lab.html
- 1% – https://en.wikipedia.org/wiki/Urine_test_strip
- 1% – https://en.wikipedia.org/wiki/Ehrlich%27s_reagent
- 1% – http://wwwchem.uwimona.edu.jm/courses/CHEM2402/Crime/Reagent_Kits.html
- 1% – http://www.urology-textbook.com/urinary-tract-infection-causes.html
- <1% – https://www.thoughtco.com/calculating-concentration-and-dilution-608178
- <1% – https://answers.yahoo.com/question/index?qid=20110506072319AAJSsLM
