- Immunoelectrophoresis refers to the process in which an antigen mixture is first separated into its component parts by electrophoresis and then tested by immunodiffusion.
- The electrophoresed antigen mixture in agarose gel allows the separation of different antigens along the gel slide, and then the lateral diffusion of an antibody in the gel occurs resulting in precipitate formation.
- Counter Current Immunoelectrophoresis is a modification of immunoelectrophoresis in which antigen and antibody move in opposite directions and form precipitates in the area where they meet in concentrations of optimal proportions.
- It is also referred to as countercurrent or crossed-over immunoelectrophoresis.
- The technique is similar to the Ouchterlony method, the only difference being that the antigen movement is facilitated by electrophoresis. It is thus also called ‘voltage facilitated double immunodiffusion’.
Interesting Science Videos
Objectives
Countercurrent immunoelectrophoresis is mostly carried out with one or more of the following objectives:
- To rapidly check any antisera for the presence and specificity of antibodies for a particular antigen.
- To detect antigens and/or antibodies in serum for diagnosis of a particular disease
Principle
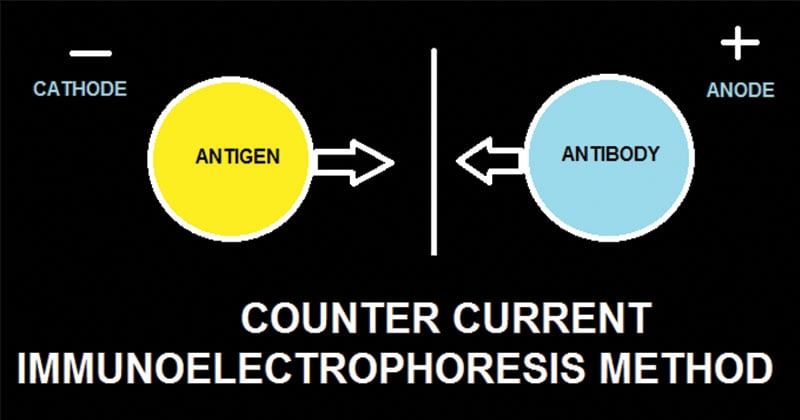
Counter-current immunoelectrophoresis depends on the movement of antigen towards the anode and of antibody towards the cathode through the agar under the electric field. The test is performed on a glass slide in agarose gel of high electro-endosmotic flow. A pair of wells is punched out where one well is filled with antigen and the other with the antibody. Electric current is then passed through the gel. The migration of antigen and antibody is greatly facilitated under the electric field, and the line of precipitation as precipitin arcs is made visible in 30–60 minutes, which indicates a positive reaction.
Materials
Agarose, Antigen, Test antiserum, Positive antiserum, Assay Buffer, Electrophoresis apparatus, Glass Slides
Procedure
- 10 ml of 1.0% Agarose (0.1 g/10 ml) in 1X Assay Buffer is prepared by heating slowly until agarose dissolves completely.
- The ends of a glass slide are marked as +ve and -ve so that when placed in the electrophoresis apparatus, the +ve mark is faced towards the anode and the negative mark faced towards the cathode.
- The glass plate or slide is placed on a horizontal surface. 5 ml of agarose is pipetted and spread onto the glass slide. It is allowed to solidify for 15 minutes.
- Wells are cut in the gel according to the template using gel puncher. The distance between the two wells is not kept more than 0.5 cm.
- The slide is placed in the electrophoresis tank and the tank filled with 1X electrophoresis buffer till the buffer just covers the gel surface.
- 10µl of antigen is added in each of the two wells towards the cathode (Negative electrode) and 10µl of positive control antiserum and test antisera in wells towards the anode (Positive Electrode).
- The power cord is connected to the electrophoretic power supply according to the convention.
- 50 V is applied and the electrophoresis is allowed to continue for about 45 minutes after the completion of which results are interpreted.
Results
- Precipitin line between the antigen and antisera wells indicate positive reaction or specific antigen-antibody reaction due to the presence of antibody specific to the antigen.
- The absence of the precipitin line indicates no reaction or the absence of any corresponding antibody-antigen.
- The presence of more than one precipitin line indicates the heterogeneity of the antibody for the antigen.
Applications
The counter-current immuno-electrophoresis has many uses:
- It is a rapid and a highly specific method for detection of both antigen and antibodies in the serum, cerebrospinal fluid, and other body fluids in the diagnosis of many infectious diseases including bacterial, viral, fungal, and parasitic.
- The test was very popular in the past for detecting various antigens such as alpha-fetoprotein in serum and capsular antigens of Cryptococcus and Meningococcus in cerebrospinal fluid.
- Still today, it is commonly used for Hepatitis B surface antigen (HBsAg), fetoprotein, hydatid and amoebic antigens in the serum, and cryptococcal antigen in the CSF.
- It is a rapid sensitive method for detecting pneumococcal capsular antigens in sputum.
Advantages
- A fast method of antigen-antibody detection (takes 30 minutes).
- More sensitive than electro-immunodiffusion (EID) because it involves simultaneous electrophoresis of the antigen and the antibody in gel in opposite directions resulting in band formation.
- Much faster and more sensitive than the double diffusion technique.
Limitations
- It is more expensive than agglutination based tests.
- It is believed to have decreased sensitivity, speed, and simplicity, then latex agglutination tests.
References
- http://www.ispybio.com/search/protocols/ie%20protocol2.pdf
- http://himedialabs.com/TD/HTI007.pdf
- Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers.
- Parija S.C. (2012). Textbook of Microbiology & Immunology.(2 ed.). India: Elsevier India.
- Sastry A.S. & Bhat S.K. (2016). Essentials of Medical Microbiology. New Delhi : Jaypee Brothers Medical Publishers.
