Colorimeter refers to a device used in colorimetry that aids in the absorption of a particular wavelength of light by a specific sample solution.
Louis J. Duboscq invented it in the year 1870. It is employed to measure how much light transmits and absorbs as it passes through a liquid. These are used to identify the color and establish the concentration of a solution. By comparing a solute’s color intensity in a solution to that of a reference solution with a known solute concentration, one can estimate the concentration of colored solute in that solution.
Interesting Science Videos
Principle of Colorimeter
When an incident light beam with intensity I0 passes through a solution, a part of the incident light is reflected (Ir) and absorbed (Ia) while the remaining incident light is transmitted (It).
i.e., Io = Ir + Ia + It
The measurement of (I0) in the colorimeter eliminates (Ir), and it is sufficient to calculate the (Ia). Using cells with the same characteristics maintains a steady amount of light reflection (Ir). Then (I0) & (It) are measured.
The operation of the colorimeter is based on Beer-Lambert’s law which states that the amount of light absorbed by a color solution is directly proportional to the solution’s concentration and the length of a light path through it.
A ∝ cl
A = ∈cl
Where,
A = Absorbance/ Optical density of the solution
∈ = Coefficient of absorption
c = Concentration of solution
l = Length of the path
A series of lenses in a colorimeter guide a beam of light with a particular wavelength through a solution as it makes its way to the measuring apparatus. This compares the colour to a current standard to examine it. The absorbance or % transmittance is then calculated using a microprocessor. By measuring the difference between the amount of light at its source and that after passing the solution, it is possible to determine the concentration of the solution and how much light will be absorbed.

Several sample solutions with known concentrations are first prepared and evaluated to ascertain the concentration of an unknown sample. Plotting the concentrations versus absorbance on a graph yields the calibration curve. To determine the concentration, the results of the unknown sample are contrasted with those of the known sample on the curve.
Instrumentation of Colorimeter
- Light Source: The source of light should produce energy with enough intensity to cover the entire visible spectrum (380-780 nm). Commonly, Tungsten lamps are used as a light source for measurement in the visible spectrum and near-infrared ranges. Halogen deuterium is suitable for measurement in the UV range (200-900 nm).
- Slit: It reduces unwanted or stray light by allowing a light beam to pass through.
- Condensing lens: Parallel beam of light emerges from condensing lens after the light passes through slit incidents on it.
- Monochromator: It filters the monochromatic light from polychromatic light, which absorbs unwanted light wavelengths and permits only monochromatic light. These are of three types: prism, grating, and glass.
- Prism: It facilitates the refraction of light when it passes from one medium to another.
- Glass: It selectively transmits light in certain ranges of wavelengths.
- Gratings: These are made of graphite, which separates light in different wavelengths.
- Cuvette (Sample cell): The monochromatic light from the filter passes through the colored sample solution placed in the cuvette. Their sizes range from square, and rectangle to round and have a fixed diameter of 1cm. These are of three types based on the substances these are made of: Glass, Quartz, and Plastic cuvette.
- Glass cuvettes are cheap and absorb light of 340 nm wavelength.
- Quartz cuvettes facilitate entry of both lights of UV and visible ranges.
- Plastic cuvettes are cheaper, easily scratched, and have shorter lifespans.
- Photocell (Photodetector): These photosensitive devices measure light intensity by converting light energy into electrical energy.
- Galvanometer: The electrical signal generated in a photocell is detected and measured by a galvanometer. It displays optical density (OD) and percentage transmission.
Types of Colorimeters
Several types of colorimeters are as follows:
- Densitometers: Determine the density of a material.
- Spectrophotometers: Measure the spectral reflectance and transmittance of a surface.
- Tristimulus colorimeter: Employed to measure the tristimulus values of a color.
Colorimeter Operating Procedure
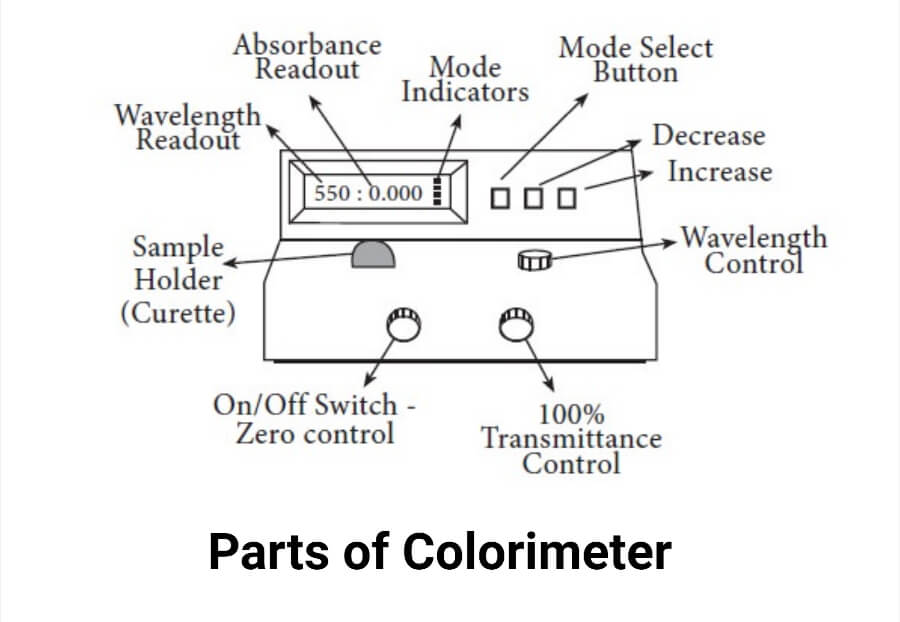
1. Switch the device on by rotating the Power Switch knob in a clockwise direction (toward the right). 15 minutes of warming up time for the colorimeter is required to stabilize the light source and the detector.
2. After the warm-up period, turn the Wavelength Control knob to the appropriate wavelength.
3. Press the MODE control key until the light next to “Transmittance” turns on to switch the display mode to transmittance.
4. Use the Zero Control knob to set the display’s T-factor to 0.0%. Make this adjustment while ensuring the sample chamber is empty and the cover is securely closed.
5. Place the blank solution in a cuvette until it reaches the top of the triangle on the side of the cuvette. To get rid of any fluids or fingerprints on the cuvette’s exterior, wipe it with a Kimwipe. Both will obstruct the light’s ability to travel and result in inaccurate readings.
6. Place the tube gently but completely into the cuvette chamber, with the vertical guide line facing in the direction of your right. The guideline on the cuvette should now be lined up with the guideline on the sample chamber by rotating the cuvette 90 degrees in a clockwise orientation. This method protects the cuvette against scratches in the light-transmitting portions. Erroneous measurements can result from scratches on the cuvette. Close the compartment’s cover.
7. Set the display to 100.0% using the Transmittance/Absorbance control knob.
8. Press the MODE control key and switch the Status Indicator light to read Absorbance. The display should indicate 0.0 if the Transmittance calibration was done correctly. No further adjusting is necessary. Use the Transmittance / Absorbance control knob to set the display to 0.0 if it does not already show that value. Switch the display back to Transmittance using the MODE key.
9. Reverse the previous process to remove the cuvette from the compartment by rotating it 90 degrees counter-clockwise before doing so. You should put the solution whose absorbance you want to test in another cuvette. Similar to before, place it inside the chamber.
a. Directly from the digital display, read the %T value.
b. Select Absorbance using the MODE key, then take the A value directly from the digital display. Select Transmittance once more.
10. Reverse the process you used to insert the cuvette to remove it from the sample compartment. Close the compartment’s cover.
Applications of Colorimeter
- It is employed in the printing industries to evaluate the caliber of printing ink and paper.
- These are used in the food and food processing industries.
- It is frequently used in laboratories and hospitals to determine the biochemical composition of samples like blood, urine, cerebral spinal fluid, plasma, serum, etc.
- It is used in the textile and paint industries.
- Diamond dealers also examine the visual characteristics of priceless stones using a colorimeter.
- The instrument is also employed in cosmetology to measure the UV protection level of skin-care products.
- They are used to evaluate the water’s purity and screen for the presence of chemicals like cyanide, iron, fluorine, chlorine, molybdenum, etc.
- They are employed to evaluate the color contrast and brightness of screens on mobile devices, computers, and televisions to give people the greatest viewing experience.
- A colorimeter is also employed in the pharmaceutical sector to spot inferior goods and medications.
- Blood samples are tested using a colorimeter to determine the amount of hemoglobin present.
Advantages of Colorimeter
- A quick and affordable means of evaluating quality is the colorimeter.
- The Colorimeter makes it simple to perform a quantitative examination of colored chemicals.
- Results are available in under a second.
- Four AA batteries can be measured using a portable colorimeter between 100 and 300 times.
Disadvantages of Colorimeter
- The procedure of determining the concentration of colorless substances becomes laborious.
- Colorimeter does not function in the ultraviolet or infrared spectrum since it only measures wavelength absorbance in the visible range of light (400nm to 700nm).
- A spectrum range must be set rather than a specific wavelength to measure the absorbance.
- Measurements might be challenging on surfaces that reflect light.
Precautions
- It is best to keep the instrument’s external environment stable while measuring. For instance, it’s best to avoid flashing ambient light while measuring.
- Avoid allowing liquids, powders, or solid foreign particles to enter the measuring caliber or within the device. Avoid making contact with the instrument and colliding with it.
- It should not be utilized in humid environments or around water mist. To prevent damage to the instrument, it should be stored in a dry, cool atmosphere.
- A clean set of cuvettes should be used. Fill the cuvettes to the top of the triangular mark or two-thirds full. For the blank solution, always use the same cuvette; for the sample, always use the other cuvette.
- For heat to dissipate and wires and sockets to be easily accessible, we need ample space (at least three inches) surrounding the unit.
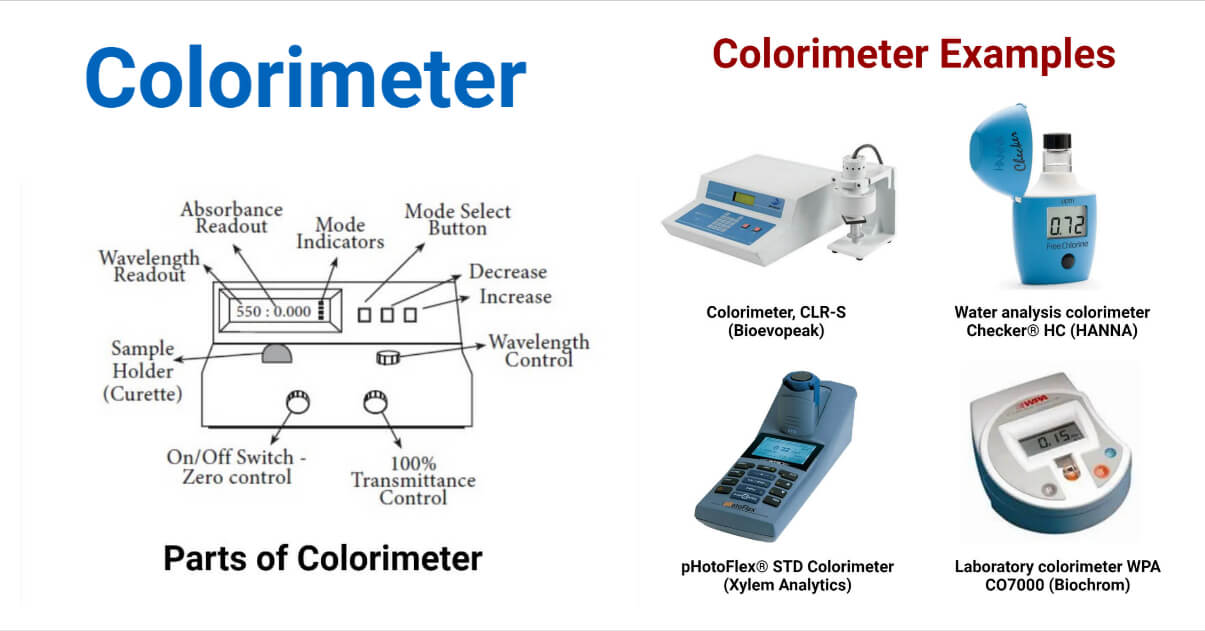
Colorimeter Examples
Laboratory colorimeter CLR-S (Bioevopeak)
- Combining the CIE standard illuminant D65 with the 10° wide viewing field, X10, Y10, and Z10 make up a sample light source.
- Automatic measuring of a substance’s reflected color in the board, powder, and grain forms.
- Printer and LCD RS232 interface.

Water analysis colorimeter Checker® HC (HANNA Instruments)
- More accurate and user-friendly than chemical test kits.
- Obtaining your results for free chlorine is easy with a single-button operation.
- Excellent for testing the environment, pools, and spas.
Environmental analysis colorimeter pHotoFlex® (Xylem Analytics)
- Turbidity measurement of 0.01 – 1,100 NTU in accordance with DIN ISO 27027
- Versatile with unique NH3 and CO2 methods
- Special combination with pH and turbidity measurement
- More than 180 programs for standard parameters
Laboratory colorimeter WPA CO7000 (Biochrom)
- The Biochrom WPA CO7000 is a portable colorimeter used by physicians and medical technicians in small and medium-sized clinics.
- Perfect for field and tropical environments
- A good fit for biological colorimetric analysis
References
- https://www.ecstuff4u.com/2021/05/advantages-disadvantages-colorimeter.html?m=1
- https://laboratorytests.org/colorimeter/
- http://www.xzbelec.com/news/industry-news/precautions-on-using-of-colorimeter.html
- https://www.tumblr.com/microamaze/134454969515/precaution-when-using-colorimeter
- https://www.amu.ac.in/department/bio-chemistry-jnmc/sops
- https://www.hunterlab.com/blog/lab-vs-lch-coordinates/
- https://infinitylearn.com/surge/chemistry/colorimeter/
- https://www.medicalexpo.com/prod/bioevopeak/product-301335-1058282.html
- https://www.medicalexpo.com/prod/hanna-instruments/product-80622-863381.html
- https://www.medicalexpo.com/prod/xylem-analytics/product-80700-925763.html
- https://www.medicalexpo.com/prod/biochrom/product-99779-644098.html
- http://www2.csudh.edu/oliver/che230/labmanual/spec20d.htm
- MK Ganesh, A Hemavathi, & YM Shivaraja Shankara. (ca. 2008). Laboratory manual for practical biochemistry (1/e). Jaypee Brothers Medical Publishers (P) Ltd. https://doi.org/10.5005/jp/books/10435_28

This is the best I have ever found.