Interesting Science Videos
What is Chemical Sterilization?
- Chemical Sterilization is the process of removal of microorganisms by the use of chemical bactericidal agents.
- Even if physical methods of sterilization are more appropriate for effective sterilization, it is not always appropriate to use for heat-sensitive materials like plastics, fiber optics, and biological specimens.
- Under such conditions, chemical either in liquid or gaseous state can be used for sterilization. However, it is crucial to ensure that the materials undergoing sterilization are compatible with the chemical being used.
- Besides, it is important to adopt safety rules in the workplace safety during the use of chemical agents.
- The chemical method of sterilization can be categorized as liquid and gaseous sterilization.
Read also: Physical methods of sterilization
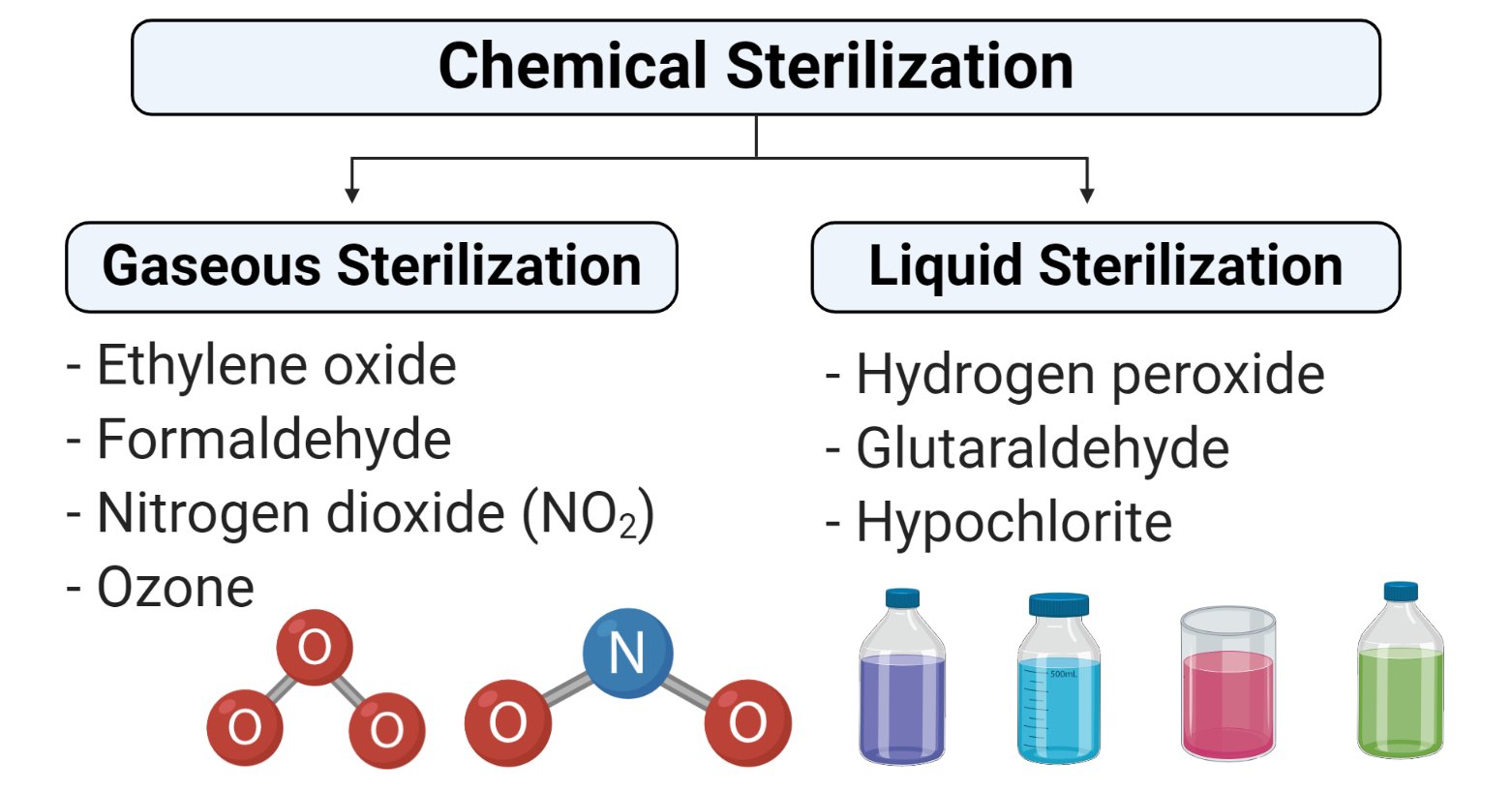
1. Gaseous Sterilization
- Gaseous sterilization involves the process of exposing equipment or devices to different gases in a closed heated or pressurized chamber.
- Gaseous sterilization is a more effective technique as gases can pass through a tiny orifice and provide more effective results.
- Besides, gases are commonly used along with heat treatment which also facilitates the functioning of the gases.
- However, there is an issue of release of some toxic gases during the process which needs to be removed regularly from the system.
- The mechanism of action is different for different types of gases.
- Some of the common gases used for gaseous sterilization are explained below:
i. Ethylene oxide
- Ethylene oxide (EO) gas is a common gas used for chemical treatment applied to sterilize, pasteurize, or disinfect different types of equipment and surfaces because of its wide range of compatibility with different materials.
- EO treatment often replaces other sterilization techniques like heat, radiation, and even chemicals in cases where the objects are sensitive to these techniques.
- This method is a widespread method used for almost 70% of all sterilizations and around 50% for disposable medical devices.
- The mechanism of antimicrobial action of this gas is assumed to be through the alkylation of sulphydryl, amino, hydroxyl, and carboxyl groups on proteins and imino groups of nucleic acids.
- EO treatment is usually conducted at the temperature range of 30-60°C for several hours which aids in the activity of the gas.
- The efficacy of the gas depends on the concentration of gas available for each article which is greatly assisted by the good penetrating nature of the gas, which diffuses readily into many packaging materials including rubber, plastics, fabric, and paper.
- Ethylene oxide kills all known microorganisms, such as bacteria (including spores), viruses, and fungi (including yeasts and molds), and is compatible with almost all materials even when repeatedly applied.
- This process, however, is not without drawbacks as the level of gas in the sterilizer goes on decreasing due to absorption, and the treated articles need to undergo a process of desorption to remove the toxic residual wastes.
- Organisms are more resistant to ethylene oxide treatment in a dried state, as are those protected from the gas by inclusion in crystalline or dried organic deposits.
ii. Formaldehyde
- Formaldehyde is another important highly reactive gas which is used for sterilization.
- This gas is obtained by heating formalin (37%w/v) to a temperature of 70-80°C.
- It possesses broad-spectrum biocidal activity and has found application in the sterilization of reusable surgical instruments, specific medical, diagnostic and electrical equipment, and the surface sterilization of powders.
- Formaldehyde doesn’t have the same penetrating power of ethylene oxide but works on the same principle of modification of protein and nucleic acid.
- As a result of the low penetrating power, its use is often limited to paper and cotton fabrics.
- Formaldehyde can generally be detected by smell at concentrations lower than those permitted in the atmosphere and thus can be detected during leakage or other such accidents.
iii. Nitrogen dioxide (NO2)
- Nitrogen dioxide is a rapid and effective sterilant that can be used for the removal of common bacteria, fungi, and even spores.
- NO2 has a low boiling point (20°C) which allows a high vapor pressure at standard temperature.
- This property of NO2 enables the use of the gas at standard temperature and pressure.
- The biocidal action of this gas involves the degradation of DNA by the nitration of phosphate backbone, which results in lethal effects on the exposed organism as it absorbs NO2.
- An advantage of this gas is that no condensation of the gas occurs on the surface of the devices because of the low level of gas used and the high vapor pressure. This avoids the need for direct aeration after the process of sterilization.
iv. Ozone
- Ozone is a highly reactive industrial gas that is commonly used to sterilize air and water and as a disinfectant for surfaces.
- Ozone is a potent oxidizing property that is capable of destroying a wide range of organisms including prions, without the use of hazardous chemicals as ozone is usually generated from medical-grade oxygen.
- Similarly, the high reactivity of ozone allows the removal of waste ozone by converting the ozone into oxygen by passing it through a simple catalyst.
- However, because ozone is an unstable and reactive gas, it has to be produced on-site, which limits the use of ozone in different settings.
- It is also very hazardous and thus only be used at a concentration of 5ppm, which is 160 times less than that of ethylene oxide.
Read also: Physical methods of sterilization
2. Liquid Sterilization
- Liquid sterilization is the process of sterilization which involves the submerging of equipment in the liquid sterilant to kill all viable microorganisms and their spores.
- Although liquid sterilization is not as effective as gaseous sterilization, it is appropriate in conditions where a low level of contamination is present.
- Different liquid chemicals used for liquid sterilization includes the following:
i. Hydrogen peroxide
- Hydrogen peroxide is a liquid chemical sterilizing agent which is a strong oxidant and can destroy a wide range of microorganisms.
- It is useful in the sterilization of heat or temperature-sensitive equipment like endoscopes. In medical applications, a higher concentration (35-90%) is used.
- H2O2 has a short sterilization cycle time as these cycles are as short as 28 minutes where ethylene oxide has cycles that as long as 10-12 hours.
- However, hydrogen peroxide has drawbacks like low material compatibility, lower capacity of penetration, and associated health risks.
- Vaporized hydrogen peroxide (VHP) is used to sterilize largely enclosed and sealed areas, such as entire rooms and aircraft interiors.
ii. Glutaraldehyde
- Glutaraldehyde is an accepted liquid sterilizing agent which requires comparatively long immersion time. For the removal of all spores, it requires as long as 22 hours of immersion time.
- The presence of solid particles further increases the immersion time.
- The penetration power is also meager as it takes hours to penetrate a block of tissues.
- The use of glutaraldehyde is thus limited to certain surfaces with less contamination.
iii. Hypochlorite
- Hypochlorite solution, which is also called liquid bleach, is another liquid chemical that can be used as a disinfectant, even though sterilization is difficult to obtain with this chemical.
- Submerging devices for a short period in liquid bleach might kill some pathogenic organisms but to reach sterilization submersion for 20-24 hours is required.
- It is an oxidizing agent and thus acts by oxidizing organic compounds which results in the modification of proteins in microbes which might ultimately lead to death.
- Appropriate concentrations of hypochlorite can be used for the disinfection of workstations and even surfaces to clean blood spills and other liquids.
Read also: Physical methods of sterilization
References
- Banwart GJ (1989). Basic Food Microbiology. Chapman & Hall, New York, NY.
- Jay JM (2000). Modern Food Microbiology. CBS Publications and Distribution. Delhi.
- Adams RM and Moss MO (2008). The Royal Society of Chemistry. Cambridge.
- https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/liquid-chemical-sterilization
- https://study.com/academy/lesson/chemical-sterilization-methods.html
Sources
- 3% – https://infogalactic.com/info/Sterilization_(microbiology)
- 2% – https://basicmedicalkey.com/sterilization-procedures-and-sterility-assurance/
- 2% – http://www.pharmacy180.com/article/gaseous-sterilization—sterilization-methods-637/
- 1% – https://www.uwo.ca/animal-research/doc/bleach-sop.pdf
- 1% – https://www.sciencedirect.com/topics/nursing-and-health-professions/ozone
- 1% – https://www.ozonetech.com/sites/default/files/datasheet_-_about_ozone_v2.0_en-web.pdf
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4791376/
- 1% – https://study.com/academy/lesson/chemical-sterilization-methods.html
- 1% – https://quizlet.com/80921551/chem-131-gas-law-test-flash-cards/
- 1% – https://en.wikipedia.org/wiki/Ionizing_radiation_sterilization
- 1% – https://answers.yahoo.com/question/index?qid=20141107093415AArUsva
- <1% – https://www.slideshare.net/tamilsilambarasan/sterilization-and-disinfection-45668455
- <1% – https://www.slideshare.net/BrittoSamuel/chemical-sterilization
- <1% – https://www.sciencedirect.com/topics/immunology-and-microbiology/vaporized-hydrogen-peroxide
- <1% – https://www.osha.gov/SLTC/hazardousdrugs/controlling_occex_hazardousdrugs.html
- <1% – https://www.lenntech.de/processes/disinfection/chemical/disinfectants-sodium-hypochlorite.htm
- <1% – https://en.wikipedia.org/wiki/Vaporized_hydrogen_peroxide
- <1% – https://en.wikipedia.org/wiki/Thermal_sterilization
- <1% – https://en.m.wikipedia.org/wiki/Sterilization_(microbiology)

I understand that so much