Centrifugation is the technique of separating components where the centrifugal force/ acceleration causes the denser molecules to move toward the periphery while the less dense particles move to the center.
- The process of centrifugation relies on the perpendicular force created when a sample is rotated about a fixed point.
- The rate of centrifugation is dependent on the size and density of the particles present in the solution.
Interesting Science Videos
Principle of Centrifugation
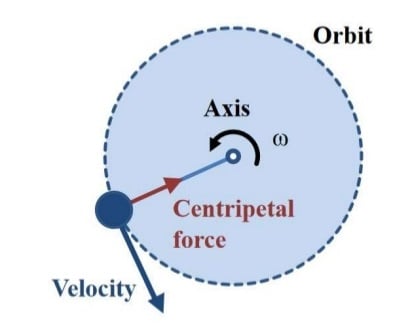
- In a solution, particles whose density is higher than that of the solvent sink (sediment), and particles that are lighter than it floats to the top.
- The greater the difference in density, the faster they move. If there is no difference in density (isopycnic conditions), the particles stay steady.
- To take advantage of even tiny differences in density to separate various particles in a solution, gravity can be replaced with the much more powerful “centrifugal force” provided by a centrifuge.
- A centrifuge is a piece of equipment that puts an object in rotation around a fixed axis (spins it in a circle), applying a potentially strong force perpendicular to the axis of spin (outward).
- The centrifuge works using the sedimentation principle, where the centripetal acceleration causes denser substances and particles to move outward in the radial direction.
- At the same time, objects that are less dense are displaced and move to the center.
- In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low- density substances rise to the top.
Types of Centrifugation
1. Analytical Centrifugation
Analytical centrifugation is a separation method where the particles in a sample are separated on the basis of their density and the centrifugal force they experience. Analytical ultracentrifugation (AUC) is a versatile and robust method for the quantitative analysis of macromolecules in solution.
Principle of Analytical Centrifugation
- Analytical centrifugation is based on the principle that particles that are denser than others settle down faster. Similarly, the larger molecules move more quickly in the centrifugal force than the smaller ones.
- Analytical ultracentrifugation for the determination of the relative molecular mass of a macromolecule can be performed by a sedimentation velocity approach or sedimentation equilibrium methodology.
- The hydrodynamic properties of macromolecules are described by their sedimentation coefficients. They can be determined from the rate that a concentration boundary of the particular biomolecules moves in the gravitational field.
- The sedimentation coefficient can be used to characterize changes in the size and shape of macromolecules with changing experimental conditions.
- Three optical systems are available for the analytical ultracentrifuge (absorbance, interference, and fluorescence) that permit precise and selective observation of sedimentation in real-time.
Steps of Analytical Centrifugation
- Small sample sizes (20-120 mm3) are taken in analytical cells to be placed inside the ultracentrifuge.
- The ultracentrifuge is then operated so that the centrifugal force causes a migration of the randomly distributed biomolecules through the solvent radially outwards from the center of rotation.
- The distance of the molecules from the center is determined through the Schlieren optical system.
- A graph is drawn from the solute concentration versus the squared radial distance from the center of rotation, based on which the molecular mass is determined.
Uses of Analytical Centrifugation
- Analytical centrifugation can be used for the determination of the purity of macromolecules.
- It can also be used for the examination of changes in the molecular mass of supramolecular complexes.
- Besides, it allows the determination of the relative molecular mass of solutes in their native state.
2. Density gradient centrifugation
Density gradient centrifugation is the separation of molecules where the separation is based on the density of the molecules as they pass through a density gradient under a centrifugal force.
Principle of Density gradient centrifugation
- Density gradient centrifugation is based on the principle that molecules settle down under a centrifugal force until they reach a medium with the density the same as theirs.
- In this case, a medium with a density gradient is employed, which either has to decrease density or increasing density.
- Molecules in a sample move through the medium as the sample is rotated creating a centrifugal force.
- The more dense molecules begin to move towards the bottom as they move through the density gradient.
- The molecules then become suspended at a point in which the density of the particles equals the surrounding medium.
- In this way, molecules with different densities are separated at different layers which can then be recovered by various processes.
Steps of Density gradient centrifugation
- A density gradient of a medium is created by gently laying the lower concentration over the higher concentrations in a centrifuge tube.
- The sample is then placed over the gradient, and the tubes are placed in an ultracentrifuge.
- The particles travel through the gradient until they reach a point at which their density matches the density of the surrounding medium.
- The fractions are removed and separated, obtaining the particles as isolated units.
Uses of Density gradient centrifugation
- Density gradient centrifugation can be applied for the purification of large volumes of biomolecules.
- It can even be used for the purification of different viruses which aids their further studies.
- This technique can be used both as a separation technique and the technique for the determination of densities of various particles.
Examples of Density gradient centrifugation
- This method was used in the famous experiment, which proved that DNA is semi-conservative by using different isotopes of nitrogen.
- Another example is the use of this technique for the isolation of the microsomal fraction from muscle homogenates and subsequent separation of membrane vesicles with a differing density.
3. Differential centrifugation
Differential centrifugation is a type of centrifugation process in which components are separately settled down a centrifuge tube by applying a series of increasing centrifugal force.
Principle of Differential centrifugation
- Differential centrifugation is based upon the differences in the sedimentation rate of biological particles of different size and density.
- As the increasing centrifugal force is applied, initial sedimentation of the larger molecules takes place.
- Further particles settle down depending upon the speed and time of individual centrifugation steps and the density and relative size of the particles.
- The largest class of particles forms a pellet on the bottom of the centrifuge tube, leaving smaller-sized structures within the supernatant.
- Thus, larger molecules sediment quickly and at lower centrifugal forces whereas the smaller molecules take longer time and higher forces.
- In the case of particles that are less dense than the medium, the particles will float instead of settling.
Steps of Differential centrifugation
- The sample solution is homogenized in the medium containing buffer.
- The sample is then placed in the centrifuge tube, which is operated at a particular centrifugal force for a specific time at a particular temperature.
- By the end of this operation, a pellet will be formed at the bottom of the tube, which is separated from the supernatant.
- The supernatant is added to a new centrifuge tube where it is centrifuged at another speed for a particular time and particular temperature.
- Again, the supernatant is separated from the pellets formed.
- These steps are continued until all particles are separated from each other.
- The particles can then be identified by testing for indicators that are unique to the specific particles.
Uses of Differential centrifugation
- Differential centrifugation is commonly used for the separation of cell organelles and membranes found in the cell.
- It can also be used for low-resolution separation of the nucleus.
- As this technique separates particles based on their sizes, this can be used for the purification of extracts containing larger-sized impurities.
4. Isopycnic centrifugation
Isopycnic centrifugation is a type of centrifugation where the particles in a sample are separated on the basis of their densities as centrifugal force is applied to the sample.
Principle of Isopycnic centrifugation
- Isopycnic centrifugation is also termed the equilibrium centrifugation as the separation of particles takes place solely on the basis of their densities and not on their sizes.
- The particles move towards the bottom, and the movement is based on the size of the particles. And, the flow ceases once the density of the particle becomes equal to the density of the surrounding medium.
- The density in the gradient increases as we move down the tube towards the bottom. As a result, the particles with higher densities settle down at the bottom, followed by less dense particles that form bands above the denser particles.
- It is considered as a true equilibrium as this depends directly on the buoyant densities and not the sizes of the particles.
Steps of Isopycnic centrifugation
- A gradient prepared with an increasing density towards the bottom of the tube is prepared. A pre-performed gradient can also be used.
- The solution of the biological sample and salt is uniformly distributed in the centrifuge tube and placed inside the centrifuge.
- Once the centrifuge is operated, a density gradient of the salt is formed in the tube.
- The particles move down the tube and settle down as they reach the region with their respective densities.
- The particles are then separated and identified using different other processes.
Uses of Isopycnic centrifugation
- Isopycnic centrifugation can be applied for the purification of large volumes of biomolecules.
- This technique can be used as a technique for the determination of densities of various particles.
5. Rate-zonal density gradient centrifugation/ Moving Zone Centrifugation
Rate-zonal density gradient centrifugation is a type of centrifugation that separates particles on the basis of their shape as size and works on the same principle of density gradient centrifugation but works in a different way. It is also called the moving zone centrifugation.
Principle of Rate-zonal density gradient centrifugation
- Rate zonal centrifugation fractionates particles by both size and shape.
- The procedure is to layer a sample in a restricted zone on top of a pre-poured density gradient. The density gradient is then centrifuged.
- All particles migrate into the density gradient because the density gradient has only densities much lower than the densities of the particles being centrifuged.
- The particles are fractionated primarily by size and shape. The larger a particle is, the more rapidly it sediments.
- The more spherically symmetrical a particle is, the more rapidly it sediments.
- The particles sediment through the gradient at a rate that is a function of their sedimentation coefficient.
- Unlike differential centrifugation where the sample is distributed throughout the medium, in rate-zonal centrifugation, the sample is initially present only on top of the gradient as a narrow band.
Steps of Rate-zonal density gradient centrifugation
- A density gradient is prepared in a centrifuge tube before applying the sample.
- The same is then layered on the top of the gradient in the form of a band.
- During centrifugation, fast-moving particles (larger in size and circular in shape) move ahead of slower particles so that different particles are separated as various bands on different parts of the gradient.
- The particles are separated on the basis of their sedimentation coefficients, and they are obtained from the bottom of the tube through a perforation.
Uses of Rate-zonal density gradient centrifugation
- Rate-zonal differential centrifugation has been used for the separation of viruses as they have components that are of different size and density that are unique to each virus.
- This method has been employed for the fractionation of RNA on sucrose gradients.
- Besides, rate-zonal differential centrifugation has also been used for the separation, purification and fractionation of DNA molecules from both viruses and bacteria.
- The fractionation of polysomes and ribosome subunits has been one of the earliest applications of this method.
6. Differential velocity (Moving Boundary) centrifugation
Differential velocity centrifugation is a type of centrifugation process in which components are separately settled down a centrifuge tube by applying a series of increasing velocities.
Principle of Differential velocity (Moving Boundary) centrifugation
- Differential centrifugation is based upon the differences in the rate of sedimentation of biological particles of different size and density.
- As the increasing speed of the rotors is applied, initial sedimentation of the larger molecules takes place.
- Further particles settle down depending upon the speed and time of individual centrifugation steps and the density and relative size of the particles.
- The largest class of particles forms a pellet on the bottom of the centrifuge tube, leaving smaller-sized structures within the supernatant.
- The pellet is then removed, and the supernatant is further centrifuged to obtain smaller particles.
- Thus, larger molecules sediment quickly and at lower velocities, whereas the smaller molecules take longer time and higher velocities.
- In the case of particles that are less dense than the medium, the particles will float instead of settling.
Steps of Differential velocity (Moving Boundary) centrifugation
- The sample solution is homogenized in the medium containing buffer.
- The sample is then placed in the centrifuge tube, which is operated at a lower rotor speed for a particular time at a particular temperature.
- By the end of this operation, a pellet will be formed at the bottom of the tube, which is separated from the supernatant.
- The supernatant is added to a new centrifuge tube where it is centrifuged at another speed for a particular time and particular temperature.
- Again, the supernatant is separated from the pellets formed.
- These steps are continued until all particles are separated from each other.
- The particles can then be identified by testing for indicators that are unique to the specific particles.
Uses of Differential velocity (Moving Boundary) centrifugation
- Differential centrifugation is commonly used for the separation of cell organelles and membranes found in the cell.
- It can also be used for low-resolution separation of the nucleus.
- As this technique separates particles based on their sizes, this can be used for the identification and comparison of particles of different sizes.
7. Equilibrium density gradient centrifugation
Equilibrium density gradient centrifugation is a modified and specialized form of density gradient centrifugation.
Principle of Equilibrium density gradient centrifugation
- Equilibrium density gradient centrifugation is based on the principle that particles in a solution are separated on the basis of their densities.
- In this case, the particles move through the density gradient and stop in a region where the density of the medium is equal to the density of the particle.
- At this point, the centrifugal force acting on the particle is equal to the buoyant force pushing the particles up. As a result, the particles cease to move and can be separated into different layers.
- The density in the gradient increases as we move down the tube towards the bottom. As a result, the particles with higher densities settle down at the bottom, followed by less dense particles that form bands above the denser particles.
Steps of Equilibrium density gradient centrifugation
- A gradient prepared with an increasing density towards the bottom of the tube is prepared. A pre-performed gradient can also be used.
- The solution of the biological sample and salt is uniformly distributed in the centrifuge tube and placed inside the centrifuge.
- Once the centrifuge is operated, a density gradient of the salt is formed in the tube.
- The particles move down the tube and settle down as they reach the region with their respective densities.
- The particles are then separated and identified using different other processes.
Uses of Equilibrium density gradient centrifugation
- Equilibrium density gradient centrifugation can be applied for the purification of large volumes of biomolecules.
- This technique can be used as a technique for the determination of densities of various particles.
Examples of Equilibrium density gradient centrifugation
- This has been used in experiments performed by Meelson and Stahl to determine the densities of different DNA molecules based on where they reached on the density gradient.
8. Sucrose gradient centrifugation
Sucrose gradient centrifugation is a type of density gradient centrifugation where the density gradient is formed of sucrose by changing the concentration of sucrose.
Principle of Sucrose gradient centrifugation
- Sucrose gradient centrifugation is based on the principle that molecules settle down under a centrifugal force until they reach a medium with the density the same as theirs.
- In this case, a medium with sucrose gradient is employed, which either has a lower density at the top and higher density at the bottom.
- Molecules in a sample move through the medium as the sample is rotated creating a centrifugal force.
- The more dense molecules begin to move towards the bottom as they move through the density gradient.
- The molecules then become suspended at a point in which the density of the particles equals the surrounding medium.
- In this way, molecules with different densities are separated at different layers which can then be recovered by various processes.
Steps of Sucrose gradient centrifugation
- A density gradient of sucrose is created by gently laying the lower concentration of sucrose over the higher concentrations in a centrifuge tube.
- The sample is then placed over the gradient, and the tubes are placed in an ultracentrifuge.
- The particles travel through the gradient until they reach a point at which their density matches the density of the surrounding medium.
- The fractions are removed and separated, obtaining the particles as separated units.
Uses of Sucrose gradient centrifugation
- Sucrose gradient centrifugation is a powerful technique for the separation of macromolecules like DNA and RNA.
- This has also been used for the analysis of protein complexes and to determine the density as well as the size of various other macromolecules.
Applications of Centrifugation
- To separate two miscible substances
- To analyze the hydrodynamic properties of macromolecules
- Purification of mammalian cells
- Fractionation of subcellular organelles (including membranes/membrane fractions) Fractionation of membrane vesicles
- Separating chalk powder from water
- Removing fat from milk to produce skimmed milk
- Separating particles from an air-flow using cyclonic separation
- The clarification and stabilization of wine
- Separation of urine components and blood components in forensic and research laboratories
- Aids in the separation of proteins using purification techniques such as salting out, e.g. ammonium sulfate precipitation.
References
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology. Eighth edition. Cambridge University Press: New York.
- Serwer, BACTERIOPHAGES: SEPARATION OF, Editor(s): Ian D. Wilson, Encyclopedia of Separation Science, Academic Press, 2000, Pages 2102-2109,
- http://trishul.sci.gu.edu.au/courses/7204BPS/Centrifugation_Lecture_2008.pdf
- http://www.biologydiscussion.com/biochemistry/centrifugation/centrifuge-introduction-types-uses-and-other-details-with-diagram/12489
- https://www.slideshare.net/poojakamble1609/principles-and-applications-of-centrifugation
- https://en.wikipedia.org/wiki/Centrifugation
- https://www.slideshare.net/khadeejaikram56/centrifugation-49732927

nicely prepared
It is a Good app please give me hai notes
Good work
Clear my doubt about the centrifugation topic 🥰🥰🥰🥰
Of course 😘😘😘
thank you do much for reliable and sufficient information.
Thats great indeed…
I’m glad reading has been made easier
very nicely explained… keep going…
thank you for the sufficient and right information.
Thank you for your advanced information and keep it up…
Good info
Thanks for your efforts
Thank you… For sufficient information