Interesting Science Videos
What is Bile Solubility Test?
Bile solubility test is a biochemical test used for the differentiation and confirmation of Streptococcus pneumoniae from other alpha-hemolytic Streptococci.
- The bile solubility test has been used as an essential test for the differentiation of S. pneumoniae as it allows the distinction between the two species S. pneumoniae and Streptococcus pseudopneumoniae, which is a challenging task.
- The test is based on the lysis of the bacterial cells in the presence of certain bile salts like sodium desoxycholate under certain conditions. The organisms that lyse are considered positive, whereas those that do not are considered negative.
- The exact working mechanism of the test is still not clearly understood, but it has been hypothesized that the lysis is brought about by the induction of autolytic enzymes.
- The bile solubility test is generally considered to be an accurate test for differentiating S. pneumoniae from other mitis group streptococci, including S. pseudopneumoniae.
- The test results from the method are, however, difficult to interpret, particularly because it is based on subjective human evaluation.
Objectives of Bile Solubility Test
- To identify and differentiate S. pneumoniae from other alpha-hemolytic Streptococci.
- To detect the ability of an organism to undergo lysis in the presence of bile salts.
Principle of Bile Solubility Test
- The bile solubility test is used to identify and distinguish S. pneumoniae from alpha-hemolytic Streptococcus spp.
- The test may be performed using a cell suspension on a slide or in a tube or by adding the reagent directly to the colony.
- The principle of bile solubility test the lysis of pneumococcal cells when sodium desoxycholate (bile salts) is applied to the colony under specific conditions of time and temperature, but other streptococci do not lyse.
- The pneumococcus has an intracellular autolytic enzyme, an amidase, which causes the organism to undergo rapid autolysis when cultivated on an artificial medium.
- The bile salts alter the surface tension of the medium and cause cell membrane rearrangement.
- The exact working mechanism of the test is not yet clearly understood; however, it has been hypothesized that the bile salts facilitate lysis of pneumococcal cells by activating the autolytic enzyme.
Microorganisms Tested
- Any alpha-hemolytic, catalase-negative, Gram-positive cocci in chains, having the characteristic central depression (flattened center) or mucoid colony morphology suggestive of S. pneumonia.
- Any Gram-positive cocci in lancet-shaped pairs from a positive blood culture.
Media, Reagents, and Supplies Used
Reagents
- Bile salts
- A 10% bile salt solution can be either purchased or prepared. Bile salt can be prepared by adding 10 grams of sodium desoxycholate in 100 ml distilled water.
- The solution should then be dispensed in small amounts to minimize contamination. The shelf life of the solution is usually 270 days.
- The storage should be done at 15 to 30°C. The storage of the reagent at cold temperatures might result in the thickening of the reagent.
- 0.85% NaCl (sterilized)
- Broth culture medium (e.g. BHI)
Supplies
- Loops
- Test tubes or slide
- Pipettes
Procedure of Bile Solubility Test
Bile solubility test can be performed either via the test tube method or direct plate method or direct slide blood culture method.
Test tube method
- About 0.5 ml of sterile saline or suitable broth is dispensed into a small test tube.
- A heavy suspension of the organism is prepared in the saline (equivalent to no.1 McFarland standard). The suspension is then shaken by hand or on a vortex to form a uniform suspension.
- The suspension is divided into two tubes, one labeled “TEST” and the other labeled “CONTROL.”
- Two to five drops of bile reagent are dispensed into both the tubes marked “TEST” and “CONTROL.” Both the tubes are gently mixed.
- The tubes are then incubated for three hours at 35°C, checking hourly for clearing, or each tube can be examined by Gram stain or methylene blue wet mount for lysis of cells at an interval of 15 minutes.
Direct plate method
- A drop of bile reagent is placed near a suspected 18- to 24 hr colony.
- The drop is then gently rolled over several representative colonies by tilting the plate. The colonies should not be dislodged with the bile reagent.
Note: Do not touch the agar surface with the tip of the dropper of bile reagent.
- The plate is kept right side up and is incubated at 35°C for 15-30 minutes or until the drop has evaporated. The plate can also be placed on a heat block as a substitute for the use of an incubator.
- The flattening of the colony is observed. Proper observation should be made to ensure that the colony does not simply float away.
Direct slide blood culture test
- One drop of blood culture broth is added to 1 drop of bile reagent on a glass slide and allowed to dry.
- As a control, one drop of broth blood culture is added to 1 drop of water and allowed to dry.
- The resulting suspension is Gram stained and examined for cocci.
Quality Control
As a form of quality control for the bile solubility test, two different organisms can be taken as a positive and negative control.
| Control | Incubation | Results |
| Streptococcus pneumoniae | Aerobic incubation for 24-48 hours at 33-37°C. | Growth positive; colonies dissolve after about 30 minutes of the addition of bile reagent. |
| Streptococcus mutans | Aerobic incubation for 24-48 hours at 33-37°C. | Growth positive; colonies remain intact after about 30 minutes of the addition of bile reagent. |
Result interpretation of Bile Solubility Test

Test tube method
- Bile solubility is demonstrated in the test tube method as a clearing or loss of turbidity, when compared to the “CONTROL” tube, within 3 hours. It can also be indicated by the lysis of cells when observed microscopically.
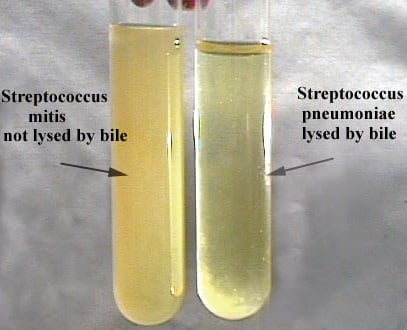
Direct slide blood culture method
- If all the cocci in the smear are entirely lysed, and the control smear shows intact bacteria, the organism is bile soluble.
Direct plate method
- Bile solubility is demonstrated as disintegration or flattening of the colony within 30 min, leaving an area of alpha-hemolysis where the colonies were located.
- A negative result is demonstrated when there is no change in the integrity of the colony within 30 min.
Reporting Results
- If either the spot or tube test demonstrates bile solubility of an alpha-hemolytic colony from a catalase-negative, lancet-shaped, Gram-positive coccus, definitively report as Streptococcus pneumoniae.
- If the test does not demonstrate bile solubility, the organism is likely a viridans group streptococcus, but a percentage of bile-resistant organisms may still be S. pneumoniae; further testing is advised from typical pneumococcal colonies.
Uses of Bile Solubility Test
- The bile solubility test is used to determine the ability of an organism to undergo lysis in the presence of bile salts.
- This test can be used for the differentiation and identification of S. pneumoniae from other alpha-hemolytic Streptococci.
- This test is a qualitative test for the differentiation of bile-soluble and bile-insoluble organisms.
Limitations of Bile Solubility Test
- Some S. pneumoniae organisms might not lyse in the presence of bile, possibly due to the loss of virulence factor or capsule. If lysis is not present, the isolate may still be S. pneumoniae. Therefore, colonies resembling S. pneumonia which are not bile soluble should be further identified using another method, such as optochin susceptibility or DNA Probe and matrix-assisted laser desorption ionization time-of-flight spectrometry (MALDI-TOF).
- Bile solubility test should only be used to differentiate S. pneumoniae from other alpha-hemolytic streptococci.
- For the greatest specificity, tube test-positive, spot test-negative strains should be confirmed with an optochin disk test or DNA probe or MALDI-TOF.
- Normal autolysis of S. pneumoniae may be inhibited by a high concentration of bile salts. Evaporation may cause the reagent to become more concentrated, thus affecting the test.
- The bile solubility test is not reliable with old cultures that have autolyzed.
- When performing the bile solubility tube test using saline or unbuffered broth, it is essential to adjust the pH to neutral before adding the reagent, in order to avoid false-negative reactions.
- When testing using the plate method, care must be taken not to dislodge the colony being tested, which lead to false-positive results. If the direct plate is difficult to interpret, the test should be repeated using the tube or slide method.
- Storage of the reagent at cold temperatures can cause it to thicken. The reagent bottle should be warmed in a 37°C incubator to liquefy the reagent before use.
References and Sources
- Biochemical Tests for the Identification of Aerobic Bacteria. (2016). Clinical Microbiology Procedures Handbook, 3.17.1.1–3.17.48.3.DOI:10.1128/9781555818814.ch3.17.1
- Slotved, H. C., Facklam, R. R., & Fuursted, K. (2017). Assessment of a novel bile solubility test and MALDI-TOF for the differentiation of Streptococcus pneumoniae from other mitis group streptococci. Scientific reports, 7(1), 7167. https://doi.org/10.1038/s41598-017-07772-x
- Tille, P.M., et al. Bailey and Scott’s Diagnostic Microbiology, V. Mosby Company, St. Louis, MO.
- 6% – https://microbiologynotes.com/bile-solubility-test-principle-procedure-result-interpretation-examples-and-limitation/
- 4% – http://www.apsi.it/public/ufiles/smi/tp5_3_en_141111.pdf
- 2% – https://catalog.hardydiagnostics.com/cp_prod/content/hugo/BileSpotRgnt.htm
- 2% – http://www.nature.com/articles/s41598-017-07772-x
- 1% – https://www.sciencedirect.com/topics/immunology-and-microbiology/optochin
- 1% – https://www.onlinebiologynotes.com/bile-solubility-test-principle-procedure-and-result/
- 1% – https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU21206.pdf
- 1% – http://www.keydiagnostics.com.au/clinical-testing-2/products-by-organism/bile-spot-reagent-detail
- <1% – https://www.sciencedirect.com/topics/neuroscience/lysis
- <1% – https://www.researchgate.net/publication/274261127_Current_methods_for_capsular_typing_of_Streptococcus_pneumoniae
- <1% – https://www.researchgate.net/publication/14534749_Identification_classification_and_clinical_relevance_of_catalase-negative_Gram-positive_cocci_excluding_the_Streptococci_and_Enterococci
- <1% – https://www.justanswer.com/medical/bcstf-blood-infection-gram-positive-cocci-pairs.html
- <1% – https://microbeonline.com/bile-solubility-test-principle-procedure-expected-result-and-quality-control/
