Beta-oxidation is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA. Acetyl-CoA enters the citric acid cycle while NADH and FADH2, which are co-enzymes, are used in the electron transport chain. It is referred as “beta oxidation” because the beta carbon of the fatty acid undergoes oxidation to a carbonyl group.
A key metabolic process that breaks down fatty acids and produces ATP and other compounds rich in energy is beta-oxidation. This activity takes place in cells’ mitochondria and is crucial for maintaining energy balance, especially during fasting or vigorous exercise. We shall investigate the mechanism, control, and relevance of beta-oxidation in this article, focusing on its role in energy generation and metabolic diseases. The majority of the molecules that make up the human body’s stores of energy are fatty acids. They are typically produced in the liver from dietary fats. Long hydrocarbon chains with a carboxyl group at one end make up fatty acids.
They can have one or more double bonds or be saturated (no double bonds). A large energy reserve known as adipose tissue contains triglycerides, which are fatty acids. The triglycerides that have been stored are converted into fatty acids and glycerol when energy demands rise, such as during fasting or physical activity. Energy generation depends on the following breakdown of fatty acids via beta-oxidation.
Interesting Science Videos
Mechanism of Beta-oxidation of Fatty Acid
Beta-oxidation of fatty acids occurs in several stages, with each stage taking place in specific tissues within the body. Let’s explore the stages and tissues involved in the beta-oxidation process.
Stage 1: Activation and Transport of Fatty Acids
The first stage of beta-oxidation involves the activation and transport of fatty acids. This step occurs in the cytoplasm of cells.
1. Activation
- Fatty acids, whether derived from dietary intake or adipose tissue mobilization, undergo activation before entering the mitochondria.
- In this process, fatty acyl-CoA synthetase enzymes activate fatty acids by coupling them with coenzyme A (CoA).
- This reaction requires the hydrolysis of ATP, resulting in the formation of fatty acyl-CoA molecules.
2. Transport
- The activated fatty acyl-CoA molecules are transported across the mitochondrial membrane to enter the mitochondrial matrix, where beta-oxidation takes place.
- This transport is facilitated by a specific transport protein called carnitine palmitoyltransferase I (CPT-I), located in the outer mitochondrial membrane.
- CPT-I catalyzes the exchange of CoA with carnitine, allowing the fatty acyl-carnitine to traverse the mitochondrial membrane.
- Once inside the mitochondrial matrix, another enzyme, carnitine palmitoyltransferase II (CPT-II), converts the fatty acyl-carnitine back into fatty acyl-CoA, ready for beta-oxidation.
Stage 2: Beta-Oxidation Cycle
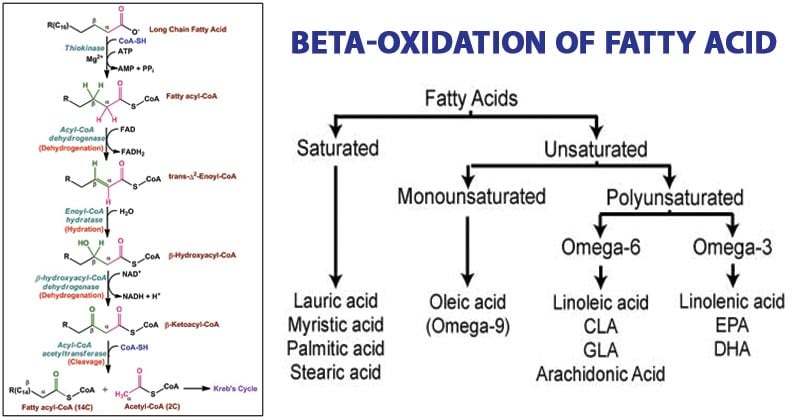
The second stage involves the actual beta-oxidation cycle, where fatty acids undergo a series of reactions to generate acetyl-CoA units. This stage occurs within the mitochondrial matrix.
1. Oxidation: The fatty acyl-CoA undergoes a series of oxidation reactions, resulting in the removal of two carbon atoms in the form of acetyl-CoA. The first step involves the oxidation of the fatty acyl-CoA by acyl-CoA dehydrogenase, which introduces a double bond between the alpha and beta carbons of the fatty acid chain. This generates trans-enoyl-CoA.
2. Hydration: The trans-enoyl-CoA molecule undergoes hydration catalyzed by enoyl-CoA hydratase, also known as crotonase. This step adds a water molecule across the double bond, resulting in the formation of L-3-hydroxyacyl-CoA.
3. Dehydrogenation: The L-3-hydroxyacyl-CoA is further oxidized by L-3-hydroxyacyl-CoA dehydrogenase, generating 3-ketoacyl-CoA. This step involves the transfer of electrons to NAD+, producing NADH.
4. Thiolytic Cleavage: The final step of the beta-oxidation cycle involves the cleavage of the 3-ketoacyl-CoA by beta-keto thiolase. This cleavage generates acetyl-CoA and a shorter fatty acyl-CoA chain, which is two carbons shorter than the original fatty acid. The shorter fatty acyl-CoA then re-enters the beta-oxidation cycle, repeating the series of reactions until the entire fatty acid is oxidized.
Stage 3: Generation of ATP and Metabolic Intermediates
- The acetyl-CoA molecules generated through beta-oxidation can enter the citric acid cycle (also known as the Krebs cycle or TCA cycle) to produce ATP through oxidative phosphorylation.
- This stage occurs in the mitochondrial matrix. The acetyl-CoA molecules derived from beta-oxidation enter the citric acid cycle, where they undergo a series of reactions, producing reducing equivalents (NADH and FADH2) and GTP (which can be converted to ATP).
- These reducing equivalents are utilized in the electron transport chain to generate ATP through oxidative phosphorylation.
Tissues Involved in Beta-Oxidation
Beta-oxidation occurs in various tissues within the body, depending on the specific energy demands and metabolic states.
1. Liver: The liver is a significant site of fatty acid oxidation, particularly during fasting. It plays a crucial role in mobilizing fatty acids from stored triglycerides in response to energy needs and producing ketone bodies as an alternative fuel source.
2. Skeletal Muscle: Skeletal muscle is another important tissue for beta-oxidation. During periods of exercise or increased energy demands, skeletal muscle utilizes fatty acids as a source of fuel for ATP production.
3. Adipose Tissue: Adipose tissue primarily functions as a storage site for triglycerides. However, during energy deficit or fasting, adipose tissue releases stored fatty acids through lipolysis, which can be taken up and oxidized by other tissues for energy production.
4. Cardiac Muscle: The heart relies heavily on fatty acid oxidation for energy production, as it has a high energy demand. Beta-oxidation provides a significant portion of ATP to support the continuous contraction and relaxation of the cardiac muscle.
Beta-oxidation of fatty acids occurs in multiple stages, starting with the activation and transport of fatty acids, followed by the beta-oxidation cycle within the mitochondria. This process generates acetyl-CoA units, which can be further metabolized in the citric acid cycle to produce ATP. Different tissues, including the liver, skeletal muscle, adipose tissue, and cardiac muscle, contribute to the overall beta-oxidation process, depending on energy demands and metabolic requirements.
Transport of Acyl-CoA into Mitochondria
The transport of acyl-CoA molecules into the mitochondria for beta-oxidation involves a specialized system known as the carnitine shuttle. This shuttle ensures the efficient entry of fatty acids into the mitochondrial matrix, where beta-oxidation takes place. The process of acyl-CoA transport involves several key steps:
1. Activation of Fatty Acids
- Before fatty acids can be transported into the mitochondria, they need to be activated to form fatty acyl-CoA.
- This activation occurs in the cytoplasm of cells and is catalyzed by enzymes called fatty acyl-CoA synthetases.
- Fatty acyl-CoA synthetases activate fatty acids by coupling them with coenzyme A (CoA), forming fatty acyl-CoA molecules.
2. Formation of Acylcarnitine
- Once the fatty acids are activated to form fatty acyl-CoA, they cannot directly cross the mitochondrial inner membrane due to its impermeability to these large molecules.
- Instead, the fatty acyl-CoA is converted into acylcarnitine through a reaction catalyzed by the enzyme carnitine palmitoyltransferase I (CPT-I). CPT-I is located on the outer mitochondrial membrane and facilitates the transfer of the acyl group from CoA to carnitine, forming acylcarnitine.
- This reaction is accompanied by the release of free CoA.
3. Transport of Acylcarnitine:
- Acylcarnitine, being a smaller molecule, can readily cross the mitochondrial inner membrane through a carnitine-acylcarnitine translocase (CACT).
- CACT transports acylcarnitine into the mitochondrial matrix while simultaneously transporting free carnitine out of the matrix and back to the cytoplasm.
- This exchange ensures a continuous supply of carnitine for the CPT-I-mediated formation of acylcarnitine.
4. Regeneration of Acyl-CoA:
- Once inside the mitochondrial matrix, the acylcarnitine is converted back into acyl-CoA through a reaction catalyzed by the enzyme carnitine palmitoyltransferase II (CPT-II).
- CPT-II is located on the inner mitochondrial membrane and facilitates the transfer of the acyl group from carnitine back to CoA, regenerating acyl-CoA.
- The released carnitine is then transported back to the cytoplasm to participate in subsequent rounds of fatty acid transport.
The transport of acyl-CoA into the mitochondria via the carnitine shuttle is a crucial step in facilitating beta-oxidation. It ensures that fatty acids are efficiently delivered to the site of beta-oxidation, allowing for the generation of ATP and metabolic intermediates. The regulation of this transport system, particularly the activity of CPT-I, is tightly controlled by various factors, including hormonal signals and the metabolic state of the cell. These regulatory mechanisms ensure a balanced utilization of fatty acids and maintain energy homeostasis in the body.
Regulation of Beta-Oxidation
- The management of beta-oxidation is complex and involves a number of elements that guarantee optimal energy storage and use.
- During periods of high energy demand, hormones like glucagon and adrenaline enhance beta-oxidation, but insulin suppresses it during the fed state.
- Long-chain fatty acyl-CoA molecules are transported into the mitochondria by the necessary molecule carnitine.
- The rate of beta-oxidation is determined by its availability. The rate-limiting enzyme that regulates the entrance of fatty acids into the mitochondria is carnitine palmitoyltransferase I (CPT-I).
- The proportion of malonyl-CoA to carnitine controls CPT-I. Malonyl-CoA levels rise when energy storage rises, blocking CPT-I and decreasing the transport of fatty acids into the mitochondria.
- On the other hand, malonyl-CoA levels fall after fasting or exercise, raising CPT-I activity and boosting beta-oxidation.
- The regulation of beta-oxidation is also influenced by the availability of cofactors and substrates.
- For instance, the activity of enzymes involved in beta-oxidation is dependent on the presence of NAD+, FAD, and CoA.
- Adequate levels of these cofactors ensure the efficient functioning of the pathway.
Role of Fatty Acids in Regulation of Beta-Oxidation
The supply of fatty acids plays a significant role in regulating fatty acid beta-oxidation. The availability of fatty acids for oxidation depends on various factors, including dietary intake, adipose tissue mobilization, and de novo lipogenesis. The regulation of fatty acid supply ensures the appropriate balance between energy utilization and storage in the body.
1. Dietary Intake
- The consumption of dietary fats provides a source of exogenous fatty acids for beta-oxidation.
- The composition of the diet, particularly the types of fatty acids consumed, can influence the regulation of beta-oxidation.
- Saturated fatty acids, which lack double bonds, are more easily metabolized compared to unsaturated fatty acids with one or more double bonds.
- Additionally, the length of the fatty acid chain affects its oxidation rate, with shorter-chain fatty acids being oxidized more readily.
2. Adipose Tissue Mobilization
- During periods of energy deficit or fasting, adipose tissue serves as a crucial reservoir of stored triglycerides.
- Hormonal signals, such as glucagon and adrenaline, stimulate the mobilization of fatty acids from adipose tissue through lipolysis.
- Hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) are key enzymes involved in the breakdown of triglycerides into fatty acids and glycerol.
- The released fatty acids can then be taken up by peripheral tissues, including muscle and liver, for beta-oxidation.
3. De Novo Lipogenesis
- In addition to dietary intake and adipose tissue mobilization, the de novo synthesis of fatty acids, known as de novo lipogenesis (DNL), can also influence the supply of fatty acids for beta-oxidation.
- DNL primarily occurs in the liver and is regulated by nutritional and hormonal factors.
- When dietary carbohydrate intake is high, excess glucose is converted into acetyl-CoA, which serves as a precursor for fatty acid synthesis.
- Under conditions of energy surplus, DNL can contribute to an increase in fatty acid supply for storage or oxidation, depending on the metabolic needs of the body.
- The supply of fatty acids for beta-oxidation is tightly regulated to ensure metabolic efficiency and energy homeostasis.
- Several factors influence this regulation, including hormonal signals and metabolic intermediates.
1. Hormonal Regulation
- Hormones such as insulin, glucagon, and adrenaline play crucial roles in regulating fatty acid supply and beta-oxidation.
- Insulin, released in response to high blood glucose levels, promotes glucose uptake and utilization, inhibiting lipolysis and fatty acid release from adipose tissue.
- In contrast, glucagon and adrenaline, released during fasting or exercise, stimulate lipolysis and increase the availability of fatty acids for oxidation.
2. Metabolic Intermediates
- Metabolic intermediates within the beta-oxidation pathway can feedback and regulate the supply of fatty acids.
- For instance, malonyl-CoA, an intermediate in fatty acid synthesis, inhibits the entry of fatty acids into mitochondria for beta-oxidation.
- This ensures that fatty acids are channeled toward storage when energy needs are met.
- Conversely, during conditions of energy deficit, malonyl-CoA levels decrease, relieving this inhibition and promoting the transport of fatty acids into the mitochondria for oxidation.
The regulation of fatty acid supply is essential for maintaining energy balance and metabolic flexibility. Dysregulation in this process can contribute to metabolic disorders such as obesity, insulin resistance, and fatty acid oxidation disorders. Understanding the intricate mechanisms that control the supply of fatty acids and their integration with beta-oxidation provides insights into the pathophysiology of metabolic diseases and can help develop targeted therapeutic strategies.
Significance of Beta-Oxidation
- The body’s ability to produce energy and maintain its energy balance depends heavily on beta-oxidation.
- When glucose levels are low by prolonged fasting or strenuous activity and fatty acids are the body’s main fuel source, this is very crucial.
- Acetyl-CoA, which may join the citric acid cycle and create the reducing equivalents (NADH and FADH2), is produced by the beta-oxidation of fatty acids.
- The electron transport chain uses these reducing equivalents after that to produce ATP by oxidative phosphorylation.
- Therefore, beta-oxidation makes a considerable contribution to ATP synthesis and the body’s overall energy balance.
- Additionally, beta-oxidation plays a role in a number of metabolic diseases.
- Fatty acid oxidation disorders (FAODs), a group of metabolic illnesses, can be caused by flaws in the beta-oxidation-related enzymes.
- Inefficient fatty acid breakdown causes a buildup of fatty acyl-CoA intermediates, which is a hallmark of several illnesses.
- Severe metabolic crises, such as hypoglycemia, liver failure, muscular weakness, and cardiomyopathy, can be caused by FAODs.
- Beta-oxidation has also drawn attention in relation to metabolic syndrome and obesity.
- The buildup of fatty acids in non-adipose tissues including the liver and skeletal muscles can cause insulin resistance, inflammation, and the emergence of metabolic diseases.
- This accumulation can be attributed to dysregulation of fatty acid metabolism and defective beta-oxidation.
Conclusion
A vital metabolic route that converts fatty acids into acetyl-CoA and other energy-rich molecules is the beta-oxidation of fatty acids. When glucose reserves run low due to fasting or vigorous activity, this mechanism acts as the body’s main source of energy. Hormones and cofactors play important roles in modulating the beta-oxidation process, which provides a balance between energy storage and utilization. Understanding the mechanics and importance of beta-oxidation might help us better understand how our bodies use energy, how metabolic illnesses are caused, and what treatments might be effective for associated ailments. For the purpose of controlling metabolic illnesses and improving the body’s use of energy, more study in this area is crucial.
References
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- Fatty Acid beta-Oxidation – https://lipidlibrary.aocs.org/chemistry/physics/animal-lipids/fatty-acid-beta-oxidation
- Fatty acid oxidation – https://www.abcam.com/pathways/fatty-acid-oxidation
- Beta-oxidation of fatty acids – https://www.slideshare.net/YESANNA/betaoxidation-of-fatty-acids
- Biochemistry, Fatty Acid Oxidation – https://www.ncbi.nlm.nih.gov/books/NBK556002/

It was very clear and understandable
Thank you.
It was really helpful
This made me pass through to my second year thanks alot
How to cite your reference. I want to cite your information.
Thanking you.
You can cite as Aryal S. 2018. Beta-oxidation of Fatty Acid. Microbe Notes. Accessed from: https://microbenotes.com/beta-oxidation-of-fatty-acid/
Thank full your topics sir