Antibodies, also known as immunoglobulins, are proteins produced by lymphocytes as a result of interaction with antigens. Antibodies are a part of the humoral immune of the adaptive immune system where each antibody identifies a specific antigen and protects the body against it.
- Antibodies are glycoproteins that bind to antigens with a high degree of specificity and affinity.
- B lymphocytes are stimulated by the binding of antigen, which results in the secretion of millions of antibodies in the bloodstream.
- The produced antibodies circulate through the bloodstream and neutralize antigens that are identical to those that triggered the immune response.
- The binding of antibodies to microorganisms or other such antigens can result in the microorganism being immobile or preventing them from penetrating the cells.
- Antibodies carry out two principal functions in the immune system. The first function is the recognition and binding to foreign bodies. The second more important function is to trigger the elimination of the attached foreign material.
- Since millions of antibodies are produced during an immune response, some of these remain in circulation in the blood for several months. This provides an extended immunity against the particular antigen.
- Each antibody is a Y-shaped protein where each tip of the Y contains a paratope that recognizes an epitope of a particular antigen.
- Antibodies can be classified into different classes based on different structures and functions.
Interesting Science Videos
Antibody Structure
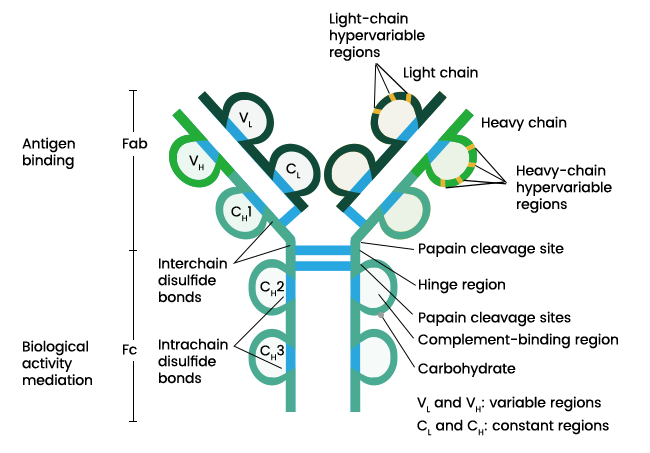
Figure: Structure of an antibody. Image Source: Sino Biological Inc.
- Antibodies are globular plasma proteins with a basic Y-shaped structure and four polypeptides.
- There are two identical heavy chains and two identical light chains connected together by disulfide bonds. The light chains consist of polypeptides of the size 22,000 Da, and the heavy chains consist of polypeptides of the size 50,000 Da.
- Each heavy chain is connected to a light chain by a disulfide bond, and the two heavy chains are connected to the light chains by two sulfide bonds.
- There are five different types of heavy chains in mammals that are designated by letters: α, δ, γ, ε and µ. There are two types of light chains designated by λ and κ.
- An antibody is composed of a variable region and a constant region. The variable region changes to various structures depending on the differences in the antigens. The constant regions are constant and do not change with antigens.
- The variable region varies between clones and is involved in antigen recognition. The constant region is conserved among clones and is required for the structural integrity and effector functions.
- The heavy and light chain in an immunoglobulin molecule consists of an amino-terminal variable region with 100-110 amino acids.
- Each light chain has one variable domain and one constant domain. The heavy chain, in turn, consists of one variable domain and three constant domains.
- The rest of the chain consists of the constant region, which limited variation that determines the light chain subtypes.
- The heavy chains in some antibodies contain a proline-rich hinge region. The hinge region separates the antigen-binding and effector domains.
- The region allows the movement of the domains enabling them to bind to antigens separated by varying distances.
Antibody Types or Classes
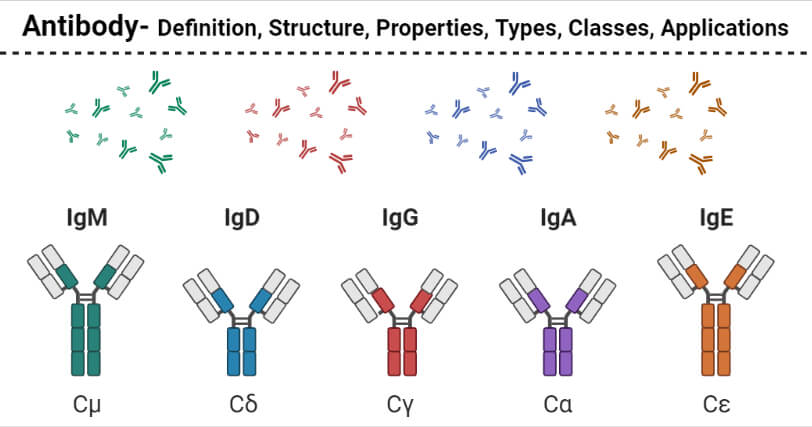
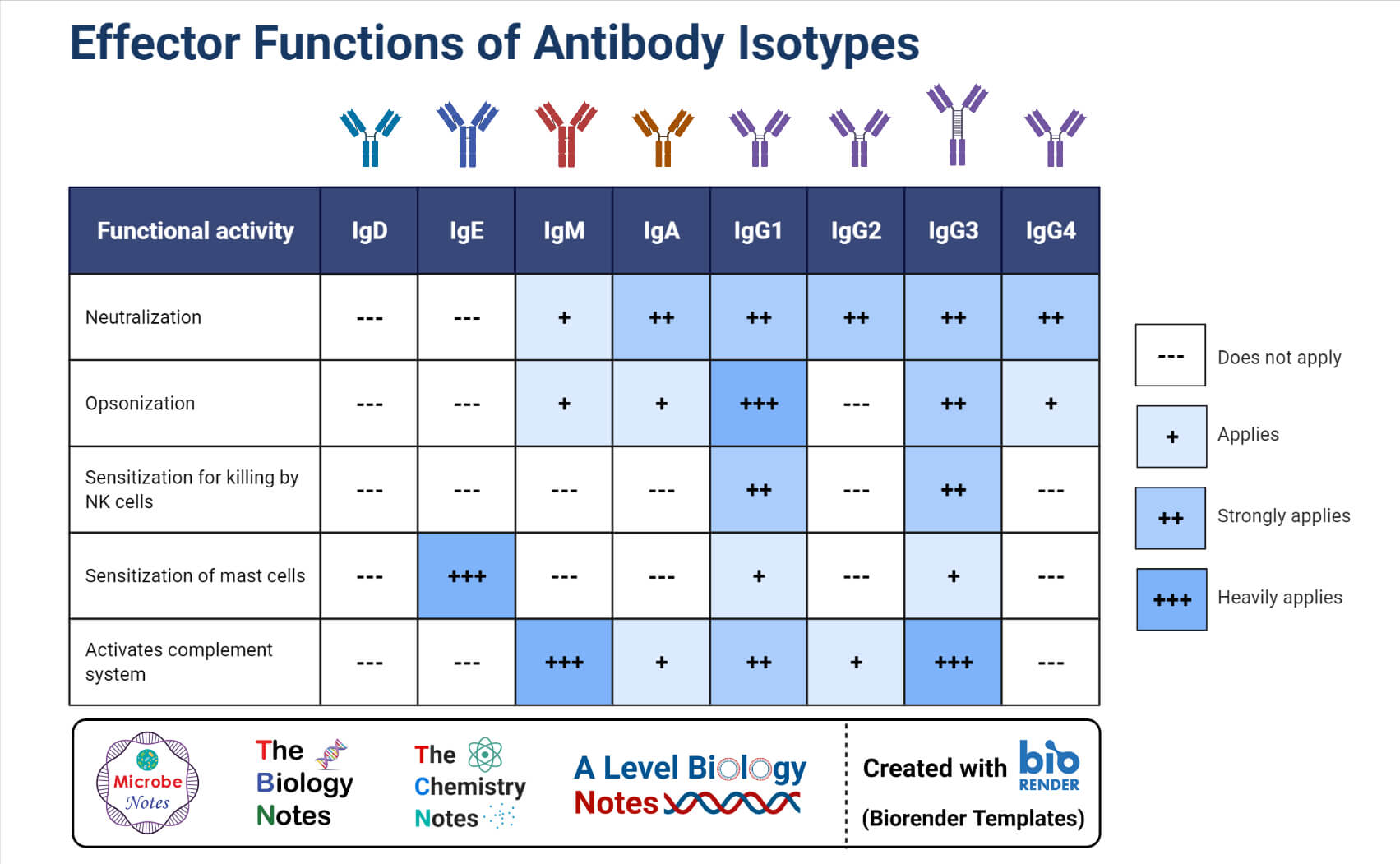
- Antibodies can be classified into five different classes; IgG, IgM, IgA, IgD, and IgE. All the antibodies have the basic four-chain antibody structure, but they have different heavy chains.
- The differences in the immunoglobulins are more pronounced in the Fc regions of the antibody, which leads to the triggering of different effector functions.
- The structural differences in the antibodies also result in differences in the polymerization state of the monomer unit.
- The following are the five different classes of antibodies;
Immunoglobulin G (IgG)
IgG is the most abundant immunoglobin, which accounts for about 80% of the total serum antibodies. The concentration of IgG in the blood is about 10mg/ml.
Structure of IgG
- The basic structure of IgG is composed of a Y-shaped protein where the Fab arms are linked to the Fc arms by an extended region of polypeptide chain called the hinge.
- The region is exposed and sensitive to attack by proteases that cleave the molecule into distinct functional units arranged in a four-chain structure.
- An IgG molecule consists of two identical γ heavy chains, usually of the size 50kDa.
- The light chains in IgG exist in two forms; κ and λ, where the κ form is more prevalent than λ, in the case of humans.
- The Fc regions of the molecule have a highly conserved N-glycosylation site in the heavy chain.
Properties of IgG
- The IgG antibodies exist in the serum in the monomeric form, and these can cross the placenta from the mother to the fetus.
- Each IgG antibody has two paratopes that bind to two different epitopes on different antigens.
- IgG has four subclasses classified on the basis of the subclasses of the γ heavy chains.
- IgG antibodies participate predominantly in secondary immune response as these are generated as a result of class switching and maturation of the response.
Subclasses of IgG
- IgG antibodies have been classified into four subclasses; IgG1, IgG2, IgG3, and IgG4.
- These are named in the order of their abundance in serum, with IgG1 being the most abundant.
IgG1
- IgG1 is the most abundant subclass of IgG antibodies with γ1 heavy chains.
- IgG1 is primarily induced by soluble protein antigen and membrane proteins but is often accompanied by lower levels of the other subclasses.
- A deficiency of IgG1 can lead to a decreased total IgG level as it is the most abundant subclass.
IgG2
- IgG2 is the second most abundant IgG in the human serum, and it is composed of γ2 heavy chains.
- IgG2 is almost entirely responsible for the response against bacterial capsular polysaccharide antigens.
- IgG2 is the only subclass of IgG antibodies that cannot cross the placenta during pregnancy.
- The deficiency of IgG2 can result in a weak defense against pathogenic microorganisms.
IgG3
- IgG3 is the third most abundant IgG occurring in human serum with the γ3 heavy chains.
- These are particularly effective in the induction of effector functions. It is a potent proinflammatory antibody with a shorter half-life.
- IgG3 is also the most effective complement activator and has a high affinity for FcR on phagocytic cells, aiding in opsonization.
IgG4
- IgG4 is the least abundant IgG antibody in human serum, which consists of γ4 subclasses of heavy chains.
- IgG4 is induced by allergens, and it is formed after repeated or long-term exposure to antigen in a non-infectious setting.
- IgG4 can cross the placenta and transfer from the mother to the fetus. IgG4 deficiencies are quite rare, but the increased levels of IgG4 in the serum have been associated with a number of problems in different organs.
Functions of IgG
- IgG antibodies provide long-term protection against various agents like bacteria, viruses, and bacterial toxins.
- IgG is one of the most potent complement activators when compared to all other antibodies.
- The binding ability of IgG to antigens is more effective as it enhances phagocytosis.
Immunoglobulin M (IgM)
IgM is the third most abundant immunoglobulin in serum, with a concentration of 1.5 mg/ml. It is the largest antibody and is the first antibody to appear in response to the initial exposure to antigen.
Structure of IgM
- IgM is secreted in a pentameric form with five distinct units, where each are composed of two µ heavy chains and two light chains.
- A J chain might be present in the hexameric form of the molecule, but it isn’t always present. The J chain is usually added just before the secretion of the pentamer as it helps in the polymerization of the monomers.
- Each of the monomers has two antigen-binding sites, resulting in 10 binding domains in a single molecule. However, all the domains cannot be occupied at the same time due to limitations in space.
- The pentameric form of IgM has a molecular weight of 900 kDa.
Properties of IgM
- IgM is the largest and the only pentameric antibody in humans. It is also the first antibody to be produced in response to the initial exposure to an antigen.
- IgM is the first immunoglobulin to be synthesized by the fetus, beginning at about 20 weeks of age.
- IgM is a pentameric molecule with 10 antigen-binding sites and 5 Fc portions held together by disulfide linkages.
- The monomeric form of IgM occurs as the major antibody receptor on the surface of B lymphocytes.
- IgM is relatively short-lived and usually disappears earlier than IgG.
- The large size of the molecules do not allow effective diffusion of the antibody, and thus, it is found in very low concentration in the intracellular fluids.
Functions of IgM
- IgM is very effective against viruses as less IgM than IgG is enough to neutralize viral infections.
- IgM is also a better agglutinin as it takes 100 to 1000 more molecules of IgG than that of IgM for the same level of agglutination.
- IgM is involved in activating the classical pathway of complement in the immune system due to the presence of two Fc regions in close proximity.
Immunoglobulin A (IgA)
IgA or sIgA is the main immunoglobulin found in the mucous membrane in the form of secretory antibodies. The concentration of IgA is found in small quantities in blood, but it is found in high concentrations in tears, saliva, and sweat
Structure of IgA
- The molecular size of IgA is 160 kDa with a four-chain monomeric structure, however, it can occur in dimeric and trimeric forms.
- The heavy chain of IgA is divisible into three constant domains, CH1, CH2, and CH3, and a variable VH domain.
- The hinge region occurs between the CH1 and CH2 domains held together by disulfide linkages.
- sIgA has a secretory component as an additional component with a polypeptide chain of 75 kDa and extracellular proteolytic fragment.
- The molecule also has a J-chain linked to the chains via disulfide bridges. The secretory and J chain facilitates the transport of IgA across epithelial cells and protects the molecule from proteolytic digestion by enzymes.
Properties of IgA
- IgA is the second most abundant immunoglobulin in humans, with a concentration of 2-4 mg/ml. It accounts for about 10-15% of the total serum concentration but is the most abundant antibody in external secretions.
- IgA is the first line of defense as it works by inhibiting bacterial and viral adhesion to epithelial cells and by neutralizing viral and bacterial toxins intracellularly.
- The secretory IgA mostly occurs in dimeric form with two monomeric units linked together by a joining peptide.
Subclasses of IgA
- IgA has been classified into two subclasses; IgA1 and IgA2.
- IgA1 is the monomeric form, and IgA2 is the dimeric or polymeric form.
- IgA1 occurs in serum IgA (about 80%), which is produced in the bone marrow and released on the mucosal surfaces.
- The IgA in most locally secreted products is polymeric with a relative release of dimeric IgA2.
- One of the most prominent differences between IgA1 and IgA2 is the hinge region which is quite extended in IgA1.
Functions of IgA
- IgA is the first line of defense as it protects the body from the entry and colonization of mucosal surfaces by different foreign particles.
Immunoglobulin D (IgD)
IgD is a monomeric antibody that occurs on the surface of immature B lymphocytes. It is produced in a secreted form in a small amount in the blood serum.
Structure of IgD
- IgD has a structural diversity throughout evolution in the vertebrates as it is flexible to complement the function of IgM.
- It is a glycoprotein with two identical δ heavy chains and two identical light chains.
- IgD found on the surface of B lymphocytes has some extra amino acids at C-terminal in order to anchor to the membrane.
- The light and heavy chains are linked together by disulfide links, but they have additional intrachain disulfide links that divide the chains into domains.
- The IgD molecule also has an extended hinge region which increases the flexibility of the molecule but decreases its resistance against proteolytic cleavage.
Properties of IgD
- IgD is found in low concentration in serum, and its exact function in the immune system is not yet clearly understood.
- It represents about 0.25% of the total serum immunoglobulins with a relative molecular mass of 185 kDa and a half-life of 2.8 days.
- It also accounts for about 1% of the proteins present in the plasma membranes of B lymphocytes. Here, it usually coexpressed with another cell surface antibody, IgM.
Functions of IgD
- The most important function of IgD is antigen receptor on B cells. It also regulates B cell function if it encounters an antigen.
- It is also secreted in some amounts in the blood, mucosal secretions, and the surface of innate immune effector cells.
Immunoglobulin E (IgE)
IgE is a type of immunoglobulin found only in mammals and synthesized by plasma cells. It occurs in a monomeric form with two ε heavy chains and two light chains.
Structure of IgE
- IgE has a typical antibody structure with ε heavy chains that have a high carbohydrate content.
- IgE has two identical antigen-binding sites consisting of both light and heavy chains and a valency of 2.
- Like all antibodies, heavy and light chains are further divided into variable and constant regions.
- The heavy chains consist of five domains, out of which one is variable, and four are constant.
Functions of IgE
- IgE is mostly associated with allergic reactions where it binds to reintroduced antigens and triggers the release of pharmacologically active agents.
- It also plays an essential role in response to allergens and antigen preparation used in desensitization immunotherapy.
Applications of Antibodies
- Antibodies can be used to treat immune deficiencies as a means of passive immunity.
- The development of monoclonal antibodies has been used to treat several diseases like multiple sclerosis, rheumatoid arthritis, and different cancers.
- Antibodies can also be used in medical diagnostics as many biochemical assays allow the detection of antibodies for the diagnosis of diseases.
- Different classes of immunoglobulins can be used to analyze the antibody profile of patients.
- Antibodies can also be used as workhorse agents in biomedical research to study the workings of different antigens and their relationship with the host.
References
- Peter J. Delves, Seamus J. Martin, Dennis R. Burton, and Ivan M. Roitt(2017). Roitt’s Essential Immunology, Thirteenth Edition. John Wiley & Sons, Ltd.
- Judith A. Owen, Jenni Punt, Sharon A. Stranford (2013). Kuby Immunology. Seventh Edition. W. H. Freeman and Company.
- Sedlacek, H.H., Gronski, P., Hofstaetter, T. et al. The biological properties of immunoglobulin G and its split products [F(ab’)2 and Fab]. Klin Wochenschr 61, 723–736 (1983). https://doi.org/10.1007/BF01497399
- Vidarsson, Gestur et al. “IgG subclasses and allotypes: from structure to effector functions.” Frontiers in immunology vol. 5 520. 20 Oct. 2014, doi:10.3389/fimmu.2014.00520

Thanks for supporting us to read more information about what ever we need keep forward
in the antibody structure explaination the point which says that heavy chain has one variable domain and one constant domain is wrong. Its the light chain which has one variable domain and one constant domain.
Please make the necessary changes.
Hi Andrew,
Thanks for the correction. We have updated the article.
Best,
Sagar
I believe it depends on which antibody. They vary, dependingon which one you are referring to.