Interesting Science Videos
Habitat of Acinetobacter baumannii
- The first Acinetobacter found in soil was discovered in 1911 by M.W. Beijerinck.
- It is associated with the aquatics environment.
- It has been recovered from soil, water, animals, and humans.
- Found in the respiratory and oropharynx secretions of infected individuals.
- Found in a hospital environment and accounts for 10% of nosocomial infections in ICU.
- It has been suggested that human skin could be the source of severe infections, such as bacteremia.
- The respiratory tract, blood, pleural fluid, urinary tract, surgical wounds, CNS, skin, and eyes may be sites for infection or colonization.
- Acinetobacter baumannii specifically targets moist tissues such as mucous membranes or areas of the skin that are exposed, either through accident or injury.
- Acinetobacter baumannii is found only rarely as part of the normal skin microflora, with one study estimating that only 3% (at most) of the population are colonized by the bacterium.
- Patients that acquire artificial devices such as catheters, sutures, ventilators, and those who have undergone dialysis or antimicrobial therapy within the past 90 days are also at risk of developing A. baumannii infections.
- Acinetobacter baumannii has the ability to form biofilms on the surface of the endotracheal tube, which may account for the relatively high levels of colonization in the lower part of the respiratory tract.
- The aerobic and opportunistic pathogen
- Survive in an inanimate object for weeks.
- Recent studies have shown that A. baumannii can be found in unsuspected reservoirs, such as food or arthropods, and additional reservoirs remain to be discovered.
- Acinetobacter species isolates have been found in a variety of food items, including raw vegetables.

Figure: Acinetobacter baumannii cultured on sheep blood agar (SBA) medium, for a 48-hour time period, at a temperature of 37°C. Image Source: CDC PHIL ID#: 17067.
Morphology of Acinetobacter baumannii
- Gram-negative rod becomes spherical at the stationary phase of growth.
- Cocobacillary.
- Size = 0.9 – 1.6 micrometers by 1.5 – 2.5 micrometers
- Grouped in pairs or in chain
- Pleomorphic.
- Non-motile (may exist twitching motility)
- Encapsulated
- Non-sporulated
- It includes porins and efflux channels which contribute to antibiotic resistance.
Genome of Acinetobacter baumannii
- A single circular chromosome
- Base Pairs = 3,976,747
- Protein Coding = 3,454
- One strain of A. baumannii called AYE contains an 86kb resistance island, called AbaR1, that is made up of 45 resistance genes.
- A. baumannii AYE has three plasmids, but none contain resistance markers.
Cultural Characteristics of Acinetobacter baumannii
- Acinetobacter species are strictly aerobic, catalase-positive, indole-negative, and oxidase-negative bacteria that are mostly encapsulated.
- Acinetobacter baumannii occurs in humans due to the ability of the bacteria to grow under the mesothermal condition at 37°C.
- The most common medium for the growth of A. baumannii includes MacConkey agar and Sheep blood agar.
- For selective isolation of the bacteria, standard laboratory media like Trypticase Soy Agar and Brain Heart Infusion Agar are used.
- Environmental strains of the bacteria can grow over a wide range of temperature with some psychrophilic strains capable of growing at 4°C and some thermophilic strains growing at 48°C.
- The growth of A. baumannii is dependent on the presence of growth factors in artificial media.
- The ability of the bacteria to grow on simple agar like MacConkey agar is due to the exploitation of various organic carbon and energy sources.
- A. baumannii can utilize compounds like aliphatic alcohols, amino acids, fatty acids, sugars, and many recalcitrant aromatic compounds like benzene, cyclohexanol, and 2,3-butanediol.
- The cultural characteristics and growth patterns of the bacteria are used as a method of identification of A. baumannii.
- Catalase positive and oxidase negative results help distinguish the bacteria from other genera of the family Neisseriaceae.
- The bacteria utilize the cytochrome system as the electron transport chain with inhibition of NADH oxidase activity with cyanide exposure.
The following are some cultural characteristics of A. baumannii on different culture media:
a. Nutrient Agar
- The colonies of A. baumannii on NA agar appear smooth and sometimes mucoid. The colonies are pale yellow to green colonies with sizes between 1-2 mm in diameter.
- In the case of environmental samples, the surface of the bacteria is smooth with entire edges and raised elevation.
b. MacConkey Agar
- The colonies of A. baumannii are small translucent and shiny that demonstrates isolated growth on the agar.
- The texture of the colonies might vary in consistency from butyrous to smooth and mucoid, depending on the source of isolation.
- The size of the colonies is between 2-3 mm, but the size might decrease on prolonged incubation as the colonies tend to shrink up in size.
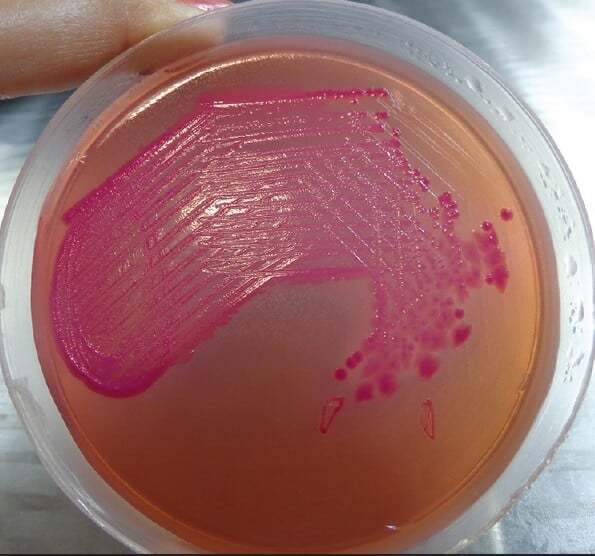
Figure: Acinetobacter baumannii on MacConkey Agar. Image Source: Medical Journal of Dr DY Patil Vidyapeeth.
Biochemical Characteristics of Acinetobacter baumannii
| Basic Characteristics | Properties (Acinetobacter baumannii) |
| Bile Solubility | Negative (-ve) |
| Capsule | Capsulated |
| Catalase | Positive (+ve) |
| Citrate | Positive (+ve) |
| Coagulase | Negative (-ve) |
| Flagella | Non-Flagellated |
| Gas | Negative (-ve) |
| Gelatin Hydrolysis | Negative (-ve) |
| Gram Staining | Negative (-ve) |
| H2S | Negative (-ve) |
| Hemolysis | Negative (-ve) |
| Indole | Negative (-ve) |
| Motility | Non-Motile |
| MR (Methyl Red) | Negative (-ve) |
| Nitrate Reduction | Negative (-ve) |
| OF (Oxidative-Fermentative) | Non- Fermentative (Oxidative) |
| Oxidase | Negative (-ve) |
| Pigment | Negative (-ve) |
| Shape | Coccobacillus |
| Spore | Non-Sporing |
| Urease | Negative (-ve) |
| VP (Voges Proskauer) | Negative (-ve) |
| Fermentation of | |
| DNase | Negative (-ve) |
| Galactose | Positive (+ve) |
| Glucose | Positive (+ve) |
| Lactate | Variable (+/-) |
| Lactose | Mostly Negative (-ve), Some Positive (+ve) |
| Malonate | Positive (+ve) |
| Maltose | Variable (+/-) |
| Mannitol | Negative (-ve) |
| Mannose | Positive (+ve) |
| Rhamnose | Positive (+ve) |
| Sucrose | Negative (-ve) |
| Xylose | Positive (+ve) |
| Enzymatic Reactions | |
| Arginine Dehydrolase | Positive (+ve) |
| Azelate | Positive (+ve) |
| Beta-Lactamase | Positive (+ve) |
| Esculin Hydrolysis | Negative (-ve) |
| Histamine | Negative (-ve) |
| Histidine | Positive (+ve) |
| Leucine | Positive (+ve) |
| Lysine | Variable (+/-) |
| Malate | Positive (+ve) |
| Phenylalanine Deaminase | Positive (+ve) |
| Tyrosine Hydrolysis | Positive (+ve) |
Virulence Factors of Acinetobacter baumannii
- Transmission of Acinetobacter and subsequent disease is facilitated by the organism’s environmental tenacity, resistance to desiccation, and evasion of host immunity.
- The virulence properties demonstrated by Acinetobacter primarily stem from evasion of rapid clearance by the innate immune system, effectively enabling high bacterial density that triggers lipopolysaccharide (LPS)–Toll-like receptor 4 (TLR4)-mediated sepsis.
- The capsular polysaccharide is a critical virulence factor that enables immune evasion, while LPS triggers septic shock.
- However, the primary driver of clinical outcomes is antibiotic resistance.
Pathogenesis of Acinetobacter baumannii
- The intrusion of a microorganism requires cell-to-cell adhesion to establish infection.
- However, the capability of A. baumannii to anchor with cells/mucosal cells is low as compared to other microorganisms.
- The reduced adhesion and invasion of A. baumannii attribute to its low virulence; however, it possesses a hydrophobic ability that provides attachment to foreign materials such as plastics used in intravascular devices.
- Outer membrane protein A (OmpA) is associated with improving adhesion, specifically to the epithelial cells of the respiratory tract. It localizes in the mitochondria and nuclei and induces expression of proapoptotic molecule cytochrome c, resulting in cell death.
- A. baumannii evades alternative complement pathway-mediated killing by neutralizing factor H, a key regulator of the alternative complement pathway, with the help of OmpA. This phenomenon is known as the serum resistance of A. baumannii.
- OmpA induces differentiation of CD4+, activation, and maturation of dendritic cells, and causes their premature apoptosis.
- Secretion of outer membrane vesicles that contain different virulence-related proteins (proteases, phospholipases, superoxide dismutase, and catalase) at the infection site accelerates the local innate immune response and ultimately leads to tissue damage.
- The outer membrane vesicles also augment biofilm formation on abiotic surfaces.
- The polysaccharide capsule plays a central role in guarding bacteria against phagocytosis by the host’s innate immune system. Lipopolysaccharides (LPSs) of A. baumannii consist of an O-antigen, the carbohydrate core, and a lipid A moiety. LPS is a chemotactic agent that recruits inflammatory cells and compels them to release their cytotoxic material.
- The ability of A. baumannii to form biofilms on biotic and abiotic surfaces is a well-studied mechanism of resistance. To survive in unfavorable conditions, it becomes metabolically inert in the deeper layers of biofilms. Poor penetration and the inability of antibiotics to act on metabolically inert bacteria augment its virulence.
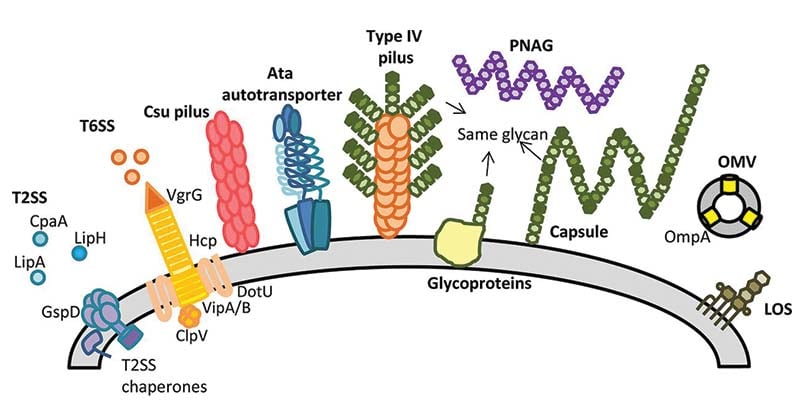
Figure: Cell surface components and secretion systems identified in Acinetobacter spp. Image Source: DOI: 10.1128/JB.00906-15.
Clinical Features of Acinetobacter baumannii
- The most frequent clinical manifestations of Acinetobacter infection are ventilator-associated pneumonia and bloodstream infections.
- Additionally, in humans, Acinetobacter can colonize skin, wounds, and the respiratory and gastrointestinal tracts.
Hospital-acquired pneumonia
- Acinetobacter pneumonia occurs predominantly in the intensive care unit (ICU) patients who require mechanical ventilation and tends to be characterized by a late-onset.
- Other clinical manifestations of Acinetobacter pneumonia are similar to those reported for hospital-acquired pneumonia in general such as dyspnea, productive cough, fever, chest pain, asymmetrical expansion of the chest, diminished resonance, egophony, etc.
Community-acquired pneumonia
- Community-acquired Acinetobacter pneumonia is typically characterized by a fulminant illness with an abrupt onset and rapid progression to respiratory failure and hemodynamic instability.
- Septic shock ensues in around one-third of patients.
Bloodstream infection
- Acinetobacter accounts for 1.5 to 2.4 percent of nosocomial bloodstream infections.
- Risk factors for Acinetobacter bloodstream infection include intensive care, mechanical ventilation, prior surgery, prior use of broad-spectrum antibiotics, immunosuppression, trauma, burns, malignancy, central venous catheters, invasive procedures, and prolonged hospital stay
- Fever may be the only manifestation of the bacteremia. Septic shock develops in up to one-third of patients with Acinetobacter bacteremia.
Endocarditis
- Acinetobacter spp are a rare cause of infective endocarditis in the native and prosthetic heart.
- Acinetobacterendocarditis is typically characterized by an acute onset with an aggressive course.
- Mortality tends to be higher in the setting of native valve endocarditis than prosthetic valve endocarditis, likely because of the low index of suspicion leading to delayed treatment in such cases.
Meningitis
- Acinetobacter is an infrequent cause of nosocomial meningitis.
- Risk factors include neurosurgical procedures, cerebrospinal fluid (CSF) leak, prior antibiotic therapy, and intracranial hemorrhage
- Mortality ranges from 20 to 30 percent; neurologic deficits in surviving patients can be severe.
- Most patients with Acinetobactermeningitis present with fever, meningeal signs, and/or seizures.
- Cerebral spinal fluid (CSF) typically demonstrates pleocytosis with neutrophilic predominance, elevated protein concentration, and a low CSF-to-serum glucose ratio. Other clinical manifestations of Acinetobactercentral nervous system infections are similar to those reported for meningitis in general.
Skin, soft tissue, and bone infection
- Acinetobactermay contaminate surgical and traumatic wounds, leading to severe soft tissue infection that can also progress to osteomyelitis
- Acinetobacter has rarely been associated with community-acquired or hospital-acquired skin infections such as cellulitis and folliculitis as well as skin abscesses and necrotizing fasciitis.
- Traumatic wound infections due to multidrug-resistant Acinetobactercomplex have been increasingly recognized after war injuries; environmental contamination of field hospitals appears to play an important role in these infections.
- Most Acinetobacterskin infections start with a skin break. Cellulitis starts as a well-demarcated edematous patch with erythema, often having a peau d’orange appearance. It then transforms into a sandpaper-like lesion characterized by numerous vesicles that might later evolve into hemorrhagic bullae.
Urinary tract infection
- The urinary tract can become colonized readily with Acinetobacter, particularly in the setting of indwelling urinary catheters.
Other infections
- Acinetobacter can cause colonization or infection of the eye. Colonization has been observed in contact lens wearers. Ocular infection can include corneal ulcers, endophthalmitis, periorbital cellulitis, and infection after penetrating trauma.
- Acinetobacter can cause nosocomial sinusitis in patients admitted to the intensive care unit; mechanical ventilation is the most important predisposing factor Acinetobacter sinusitis has been associated with the development of pneumonia, as the infected sinuses serve as reservoirs for dissemination to the lower respiratory tract.
- Acinetobacterperitonitis has been described in patients undergoing peritoneal dialysis. The most common manifestations are abdominal pain and a cloudy dialysate.
Laboratory Diagnosis of Acinetobacter baumannii
- The diagnosis of Acinetobacter infection is made by the growth of Acinetobacter from a patient specimen (eg, sputum, blood, cerebrospinal fluid) in the setting of other clinical findings that suggest an infection at that site.
- Since Acinetobacter colonization is common and treatment difficult and potentially associated with substantial toxicity, the distinction between colonization and infection, with treatment reserved for true infections, is important.
- As an example, Acinetobacter isolated from the sputum of a ventilated patient is more likely to represent colonization than infection in the absence of fever, leukocytosis, increased respiratory secretions, need for additional respiratory support, or a new abnormality on chest imaging.
- For cases of pneumonia, quantitative or semiquantitative cultures on sputum specimens may also be helpful in distinguishing between infection and colonization.
Specimens and Specimen Collection
A. baumannii has been isolated from blood, sputum, skin, pleural fluid, and urine, usually in device-associated infections.
Blood
- Collect blood by strict aseptic technique.
- Clean the rubber stopper of the blood culture bottle with an alcohol pad.
- Inoculate blood into blood culture bottles.
- Label the blood culture bottles with the date, name, and hospital number of the patient and send the specimens to the laboratory
- Keep inoculated bottles at 37oC or at room temperature.
Sputum
- Give the patient a sterile wide-mouthed screw-capped container.
- Instruct the patient to inhale deeply 2–3 times and cough out deep from the chest.
- Open the container and spit the sputum into the bottle. Avoid saliva or nasal secretions.
- Close the container for further processing.
Bronchoalveolar Lavage (BAL) and Bronchial Washings
- Collect bronchial samples by injecting a variable volume of saline through a bronchoscope channel and aspirate in 3–4 aliquots.
- Collect the aspirate in sterile disposable red-capped 40 mL wide-mouthed container or sterile bottles.
- Collect bronchial washings by aspirating small amounts of instilled saline from the large airways of the respiratory tract.
- Store the samples in sterile disposable 40 mL wide-mouthed containers or if from ICU in sterile 100 mL bottles.
Perform Gram’s staining
Acinetobacter spp. appear Gram-negative and coccobacilli in shape.
Preliminary Identification of Acinetobacter spp. by Culture Method and Propagation of Specimen Cultures
- Take samples from the collected tubes which showed Gram-negative coccobacilli in the Gram stain.
- Streak samples with a sterile disposable inoculation loop on nutrient agar, MacConkey agar, or blood agar plates.
- Incubate the inoculated plates at 37oC under aerobic conditions.
- Colony morphology on nutrient agar, blood agar, and MacConkey agar plates has to be observed and documented.
- On nutrient agar, non-mucoid, opaque, and circular colonies will be observed.
- On blood agar, nonhemolytic, opaque, circular, and gray color colonies will be observed.
- On MacConkey agar, non-lactose, fermenting, opaque, and circular colonies will be observed.
- Inoculate the biochemical reactions for preliminary identification.
- Cytochrome oxidase test (Negative)
- Mannitol motility medium (Mannitol not fermented and Non-motile)
- Triple sugar iron agar ( Alkaline butt and slant; No H2S or gas)
- Indole test (Negative for indole)
- Citrate utilization test (Citrate positive)
- Urease test (Negative)
- Inoculate the biochemicals for species identification.
- Oxidative-fermentative medium (Oxidative utilization of Glucose)
- Arginine decarboxylase test (Positive)
- Nitrate reduction test (Negative)
- Interpret the results based on positive and negative controls.
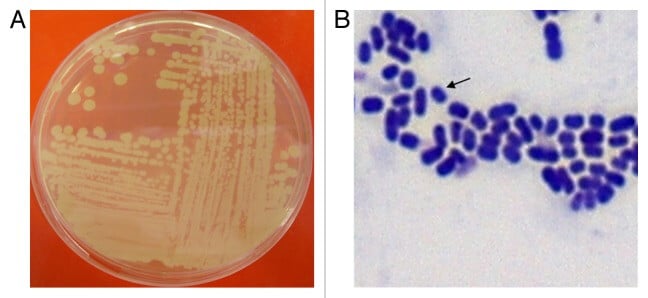
Figure A- Complex streak of Acinetobacter baumannii following overnight growth on Luria-Bertani agar at 37°C. Figure B- Gram-stain of log-phase A. baumannii cells grown in Luria-Bertani broth. Arrow indicates an individual A. baumannii cell. Image Source: Aoife Howard et al. 2012.
Treatment of Acinetobacter baumannii Infections
- Although carbapenems are effective antibiotics to treat A. baumannii infections, the rate of carbapenem-resistant A. baumannii isolates has been increasing gradually.
- Imipenem or ceftazidime combined with aminoglycosides is used for serious infections.
- Only a few effective antibiotic options are available to treat MDR A. baumannii infections. To combat MDR or pandrug-resistant (PDR) A. baumannii, which are resistant to all available antibiotics, combination therapies, including colistin/imipenem, colistin/meropenem, colistin/rifampicin, colistin/tigecycline, colistin/sulbactam, colistin/teicoplanin, and imipenem/sulbactam, have been extensively studied.
Prevention of Infection of Acinetobacter baumannii
- Acinetobacter can live on the skin and may survive in the environment for several days, which makes Acinetobacter baumannii prevention a delicate issue.
- Careful attention to infection control procedures, such as hand hygiene and environmental cleaning, can reduce the risk of transmission.
- Patients are placed in contact isolation if the pathogen was determined to be MDR.
Hand hygiene
Hand hygiene is the most fundamental, effective, and cost-effective strategy for reducing cross-infection and avoiding the spread of resistant bacteria.
Contact precaution
- The microbiology laboratory should notify clinicians in a timely and reliable way when an XDR-GNB strain is identified.
- Clinicians may implement contact precaution measures such as single room and partial separation of at least 1 m between beds for patients infected with XDR-GNB and to reduce the practice of sharing devices.
- Sphygmomanometer, stethoscope, thermometer, infusion pump, and other relevant devices should be provided specifically for patients with XDR-GNB infection
Active screening
In the ICU and other wards with highly prevalent XDR-GNB strains, patients should be screened with samples of perianal and rectal swabs for CRE, wound secretion, and nasopharyngeal region for XDR non- fermenters to promptly identify resistant bacteria by way of conventional or rapid diagnostic methods. The patients should be isolated appropriately.
Environmental surface disinfection
- The surface of the objects frequently contacted by healthcare staff and patients in the hospital environment should be disinfected regularly and completely.
- Fluorescence labeling or the ATP Hygiene Monitoring System can be used to monitor the effectiveness of disinfection and thus ensure that the transmission of resistant strains is effectively blocked.
Decolonization
Patients colonized with XDR-GNB may have a whole-body sponge bath with chlorhexidine, which is helpful for reducing catheter-related bloodstream infections.
References and Sources
- Lee, C. R., Lee, J. H., Park, M., Park, K. S., Bae, I. K., Kim, Y. B., Lee, S. H. (2017). Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Frontiers in cellular and infection microbiology, 7, 55. doi:10.3389/fcimb.2017.00055
- Asif, M., Alvi, I. A., & Rehman, S. U. (2018). Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infection and drug resistance, 11, 1249–1260. doi:10.2147/IDR.S166750.
- Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3(3):243-250. doi:10.4161/viru.19700.
- https://cmr.asm.org/content/30/1/409
- https://www.fda.gov/media/103337/download
- https://www.uptodate.com/contents/Acinetobacter-infection-epidemiology-microbiology-pathogenesis-clinical-features-and-diagnosis
- https://bestpractice.bmj.com/topics/en-gb/720
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4585070/
- https://www.sciencedirect.com/science/article/pii/S1198743X15009866
- https://www.sciencedirect.com/topics/immunology-and-microbiology/Acinetobacter-baumannii
- http://solutionsdesignedforhealthcare.com/Acinetobacter-baumannii-prevention.
