Interesting Science Videos
Wheatley Trichrome Staining definition
Wheatley Trichrome staining technique is a special permanent satin used in parasitology for the detection and identification of intestine protozoans from stool samples.
- Trichrome staining is performed on a PVA-fixed or Schaudinn’s- Preserved stool sample.
- The protozoan morphologies identified are cysts and trophozoites.
- Gomori developed this stain in 1951 and it was late modified by Wheatley in 1952 adding fixative and hydration steps to the initial procedure and formed a rapid yet simple staining technique for intestinal amoeba and flagellates.
Principle of the Wheatley Trichrome Stain
The procedure uses stool or fecal film samples that are permanently smeared to facilitate the detection and identification of cysts and trophozoites. It is very sensitive, hence it is able to detect small protozoans missed during we mount examination. The Wheatley Trichome satin is a modified stain of Gomori’s original procedure of tissues. The technique has a simple procedure, performed rapidly. It produces uniformly stained smears of the intestinal protozoans, yeasts, artifacts, and human cells. The stool sample used is fresh, or it is fixed in polyvinyl alcohol (PVA) or preserved in sodium acetate-acetic acid-formalin (SAF).
Preparation of Reagents
- Trichrome (Wheatley’s formulation)
- Chromotrope 2R…………………. 0.6 g
- Light green SF…………………… 0.3 g
- Phosphotungstic acid…………… 0.7 g
- Acetic acid (glacial)……………… 1.0 ml
- Distilled water……………………. 100.0 ml
- Add 1.0ml of acetic acid to the dry components and allow it to ripen for 3omin at room temperature. Then add 100ml of distilled water and the color of the solution changes to purple. Protect the solution from light and it should be stored in a glass or plastic bottle at room temperature. The solution has a shelf life of 24 months.
- 70% Ethanol plus iodine: To prepare a stock solution, add iodine crystals to 70% alcohol until you obtain a dark solution (1-2g/100ml). To use, dilute the stock with 70% alcohol until a dark reddish-brown color or strong tea color is obtained.
- 70% Ethanol
- 90% Acid Ethanol: Prepared by mixing 99.5 ml of ethanol with 0.5 ml of glacial acetic acid.
- 95% ethanol
- 100% ethanol
- Xylene or xylene substitute
- Mounting medium (Permount)
- Immersion oil
Procedure for Wheatley Trichrome Staining
Note: Put on hand gloves when performing the Wheatley Trichrome Stain.
Stool preparation
- Prepare the fresh stool sample. Place small samples of stool on clean sterile microscopic slides using applicator sticks. making very thin smears of the sample.
- Do not dry, and immediately place the slides in Schaudinn’s fixative and allow it to fix for 30 minutes. If the stool sample is watery, add 3 or 4 drops of PVA on the smeared slide (slide with a stool sample) and mix well, and allow it to dry for several hours at 35° – 37°C or overnight at room temperature.
Staining the smears
NOTE: PVA smear preparation is placed in iodine-alcohol (70% ethanol plus iodine) for 10 minutes. For other samples with different fixatives without mercuric chloride, omit the iodine step.
- Remove the slide from the Schaudinn’s fixative and place the slide in 70% ethanol for 5 minutes.
- Place the slide in 70% ethanol plus iodine for I minute for fresh stool samples or 5-10 minutes for PVA-preserved air-dried samples.
- Place the slide in 70% ethanol for 5 minutes.
- Place the slide in 70% ethanol for 3 minutes.
- Place the slide in Trichrome stain for 10 minutes.
- Place the slide in 90% ethanol and add acetic acid for 1-3 seconds and drain the rack immediately and proceed to the next step. Do not allow the slide to remain in this solution.
- Dip the smeared slide in 100% ethanol, this is the rinsing step.
- Place the slide in two changes of 100% ethanol for 3 minutes for each change.
- Place it in xylene for 5-10 minutes.
- Mount the smear with a coverslip using a mounting medium such as permount.
- Allow the smear to dry overnight or a minimum of 1 hour at 37°C.
- The smear is examined microscopically at 100X. Examine-in oil immersion at 200-300 fields.
Results and Interpretation of Wheatley Trichrome Stain
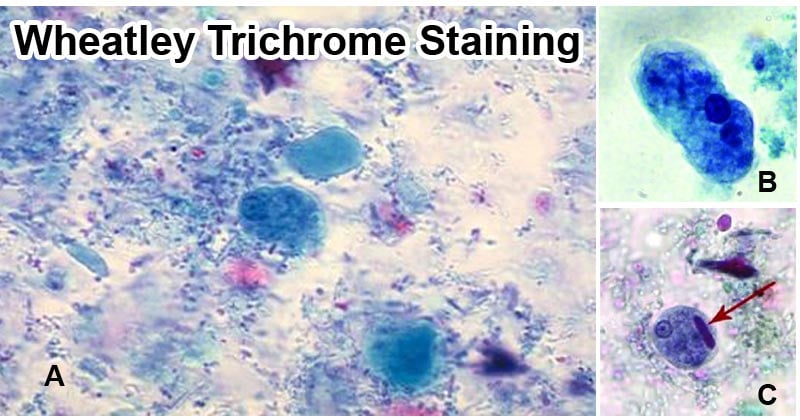
Figure: A- Wheatley’s Trichrome Stain (Image Source: Atom Scientific), B- Trophozoite of E. histolytica (Image Source: Piotr Nowak et al.). C- Cyst of E. histolytica (Image Source: Piotr Nowak et al.).
- Observe protozoan trophozoites and cysts.
- The cytoplasm of the protozoan trophozoites stains blue-green or light purple.
- The cysts appear more purple.
- The presence of yeast and human cells such as Red blood cells, polyMorphonuclear cells (PMNs), and macrophages can be identified and stained red in color.
- The nuclei and inclusion bodies also stain red with a tinged purple.
- The background stains green showing a great contrast with that of protozoans.
- Glycogen molecules are dissolved by the stain solvents and they appear as clear as the organism.
Limitations of Wheatley Trichrome Stain
- Helminth eggs and larvae can not be stained permanently by the Wheatley Trichrome Stain.
- To identify and examine protozoans, the stain requires high magnification and under oil immersion, some morphologies of the protozoans can be lost or missed.
- Some protozoans can not be identified using the Wheatley Trichrome stain such as Cryptosporidium parvum and Cyclospora cayetanensis.
- Microsporidia spores can not be seen using the Wheatley Trichrome Stained smear.
Applications of Wheatley Trichrome Stain
- For diagnosis of intestinal protozoa infections.
- For identification of parasitic morphologies.
- For identification of different yeast cells.
- For identification of human cells.
References and Sources
- 7% – https://www.cdc.gov/dpdx/diagnosticprocedures/stool/staining.html
- 3% – https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/TrichromeStainKitRgnts.htm
- 1% – https://www.newcomersupply.com/product/trichrome-stain-wheatley-modified/
- 1% – https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU40134.pdf
- 1% – https://answers.yahoo.com/question/index?qid=20110310102411AAzKALk
- 1% – http://www.med-chem.com/pages/lab_procedures/pdf/wheatleys_trichrome_stain.pdf
- <1% – https://www.austincc.edu/ddingley/MLAB1331/LabManual/LabManual.htm
