The West Nile Virus (WNV) is a mosquito-borne neurotropic virus of the family Flaviviridae. It was first reported in the West Nile province of Uganda in 1937.
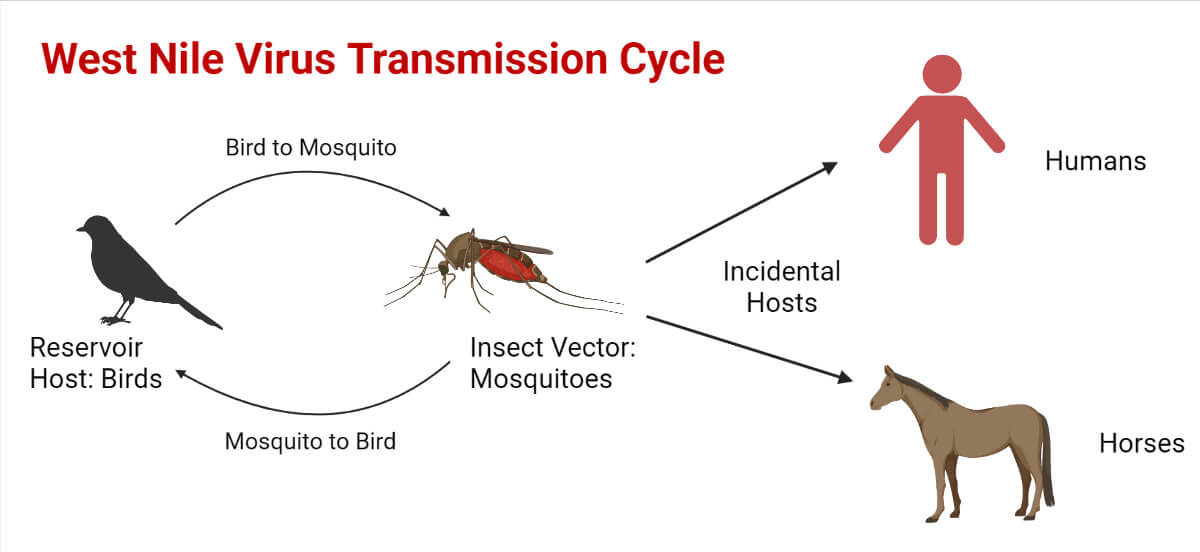
The pathogen is maintained through the mosquito-bird-mosquito (enzootic) transmission cycle with Culex mosquitoes primarily acting as the vectors, birds as the natural reservoirs, and occasional spill-over from the transmission cycle affecting humans and horses.
The infected horses as well as the humans act as “dead-end” hosts as neither of them develops sufficient viremia to transmit the virus to others.
The virus is related to other viruses causing yellow fever and dengue fever and is more closely related to viruses causing encephalitis.
Interesting Science Videos
Structure of West Nile Virus (WNV)
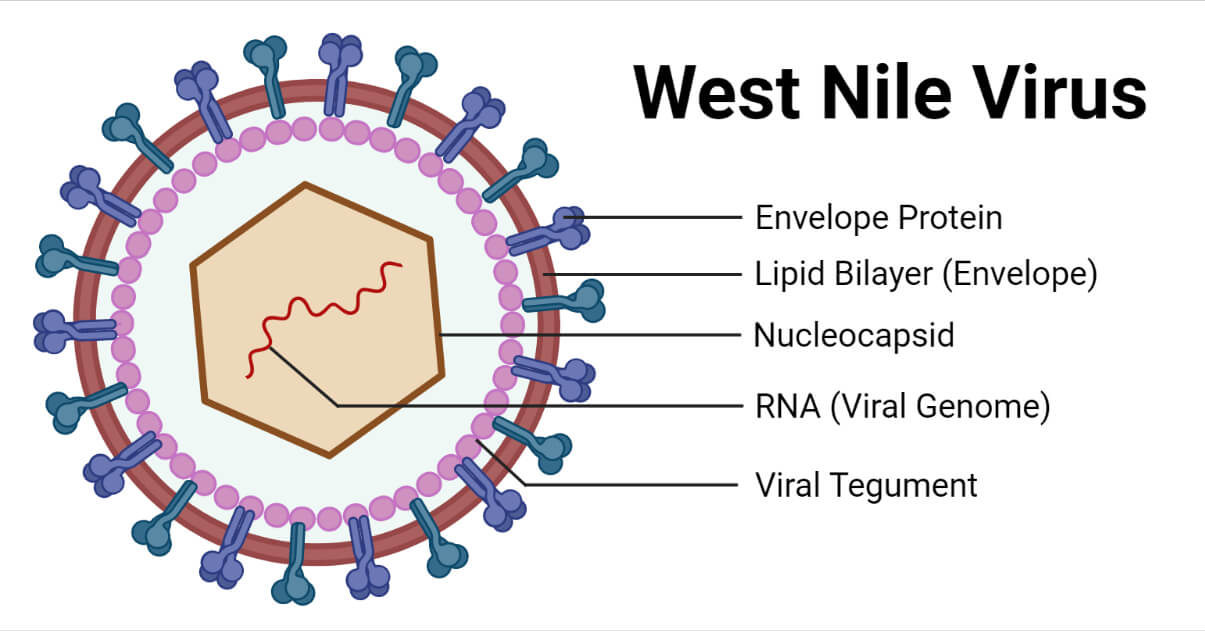
- The structure of the West Nile virus consists of a single-stranded, positive-sense RNA genome associated with the capsid to form the nucleocapsid having an icosahedral symmetry.
- The nucleocapsid is surrounded by a lipid bilayer forming the envelope. The mature virions are approximately 40-50 nm in diameter and spherical in shape.
- The glycoproteins on the envelope protrude as spikes that help in the viral attachment to the host cells.
Genome structure of West Nile Virus
- The genome of the WNV consists of single-stranded, positive-stranded RNA.
- The RNA is approximately 11kbp in size and consists of a single open reading frame.
- It lacks a polyadenylated tail in the 3’ end but instead contains noncoding regions also known as the untranslated regions (UTRs) in the 3’ and 5’ ends that aid in replication, transcription, translation, and packaging.
- The viral RNA is translated into a single polyprotein by the viral and host proteases.
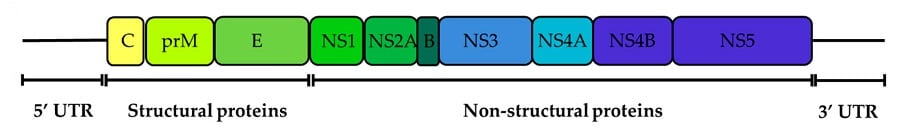
- The genome encodes for three structural (capsid, envelope, and pre membrane) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins.
- The structural proteins are encoded at the 5’ end that aid in viral entry, fusion, and encapsidation.
- The nonstructural proteins are encoded at the 3’ end with many diverse roles necessary for genome replication.
Epidemiology of West Nile Virus
- The WNV was first isolated from a febrile woman in Uganda in 1937.
- Few outbreaks of the virus were recorded until the beginning of the 1990s in human and horse populations.
- The symptoms in human populations were usually mild with rare neurologic manifestations except in the outbreaks in Israel in the 1950s and France in the 1960s that observed encephalitis in the human and horse populations.
- A number of outbreaks occurred in the 1990s in Algeria, Morocco, Tunisia, Italy, France, Romania, Israel, and Russia that caused serious human diseases including neurological manifestations and death.
- The virus was first documented in the New York City of the United States, and over the next decade, spread throughout the United States and into Canada, Mexico, and the Caribbean.
Transmission of West Nile Virus
The West Nile Virus circulates through the enzootic cycle.
The virus is transmitted to its vertebrate hosts by an infected mosquito vector during the process of blood-feeding.
Culex mosquitoes serve as the primary vectors of viral transmission whereas other species of mosquito-like Aedes can also serve as bridge vectors.
The transmission can also occur in a few numbers through:
- Blood transfusions and organ transplantation
- Exposure to the virus in the laboratories
- Mother to baby during the pregnancy, delivery, or breastfeeding
However, the WNV is not transmitted through:
- Coughing, sneezing, or touching infected people
- Touching live animals
- Handling live or dead infected birds (However, the use of gloves and plastic bags for disposing of the dead birds are recommended, and touching the dead animals with bare hands must be avoided as far as possible)
- Eating infected animals (Proper instructions must be followed while cooking the meat)
Replication of West Nile Virus
- Attachment/Adsorption
The exact receptor for the WNV is not yet clearly understood. However, a number of potential receptors for the virus include DC-SIGN, mannose receptor, and several glycosaminoglycans.
- Penetration
The virus enters the host cells through receptor-mediated endocytosis and gets transported to the endosomes.
- Uncoating
The acidification of the endosomal compartment causes conformational changes in the E protein, resulting in the fusion of the viral membrane with the membrane of the endosomal compartment. This results in the release of the viral nucleocapsid into the cytoplasm.
- Biosynthesis
The viral RNA is translated into a single polyprotein and processed. Genome replication is carried out in specific domains of viral proteins. The viral proteins cause extensive expansion and modification of the Endoplasmic reticulum. The two important domains for viral replication and protein processing are the vesicle packets (VP) and convoluted membranes (CM) respectively.
- Assembly
The viral assembly occurs at the endoplasmic reticulum from where it buds off and then enters the Golgi apparatus.
- Maturation
Viral maturation occurs in the Golgi apparatus where the prM protein of the immature virion gets cleaved and the mature viral particle is released into the cytoplasm.
- Release
The viral particle travels to the cell surface in an exocytic vesicle and is released from the cell through the process of exocytosis.
Pathogenesis of West Nile Virus
- The exact mechanism of pathogenesis of the virus in humans has been difficult to understand due to the differences between the viral strains as well as infrequent laboratory-confirmed human infections.
- Most of the knowledge regarding WNV pathogenesis comes from the study of animal models infected under controlled laboratory conditions.
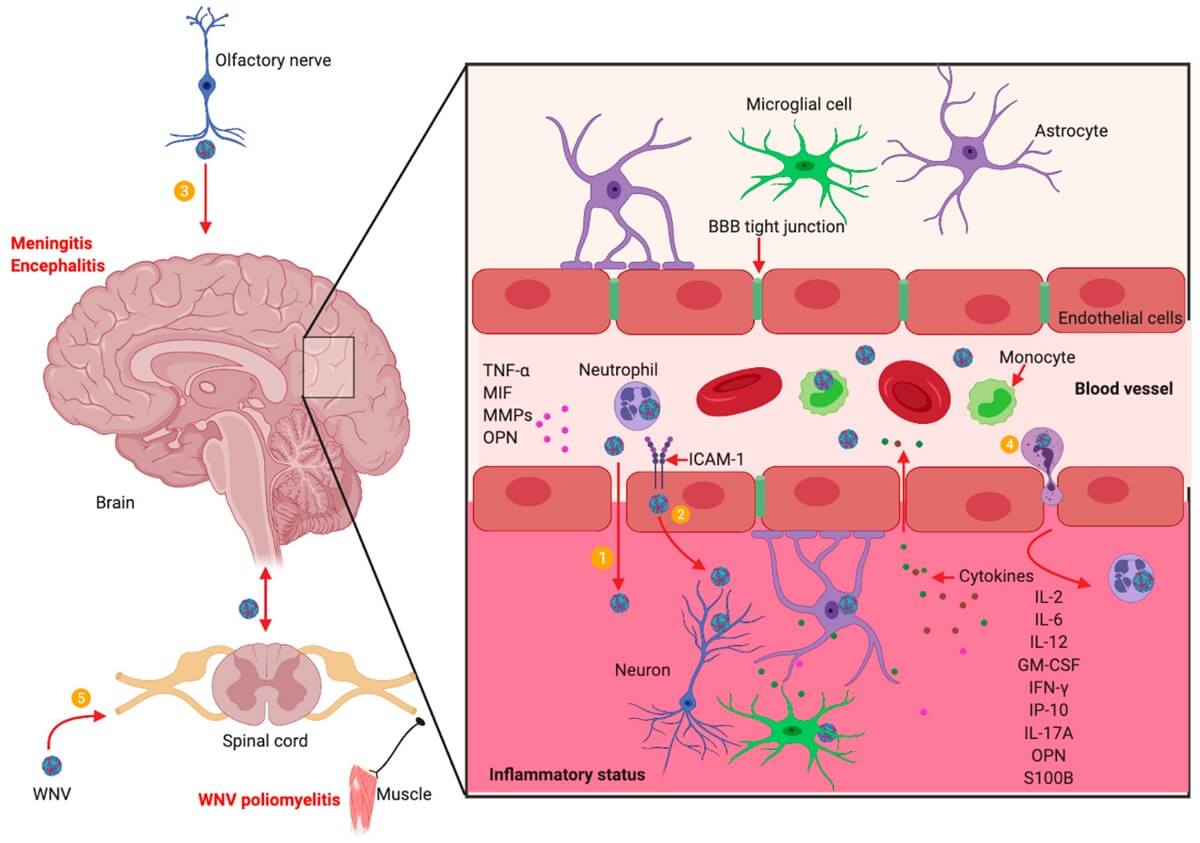
- The WNV enters the human host from an infected mosquito through its saliva during a blood meal and gets deposited in the blood and skin tissue.
- The virus then infects the neighboring dendritic cells like Langerhans cells and then travels to the lymph nodes.
- It multiplies in the tissues and causes a transient viremia that typically lasts for a few days.
- Anti-WNV IgM antibodies are produced against the virus that depletes the viral load during the viremia.
- Subsequently, the virus infects multiple organs in the body like the spleen, liver, and kidneys.
- The virus can be detected in the urine of a patient with encephalitis and the viral infection in the kidneys 8 days after the onset of the symptoms.
- The virus is able to cross the blood-brain barrier (BBB) and therefore causes neurological disorders.
- It may however pass into the CNS without disrupting the BBB.
- The WNV pathogenesis is also associated with the host’s immune response.
Clinical Manifestations of West Nile Virus
About 80% of the infected people remain asymptomatic and 20% of the infected people can develop West Nile fever or severe West Nile disease.
The incubation period is usually 3 to 14 days.
However, an extended incubation period of about 21 days has been observed among immunocompromised patients.
The common symptoms in the febrile illness are:
- Headache
- Body aches
- Fever
- Joint pain
- Vomiting
- Diarrhea
- Occasional skin rash (on the trunk of the body)
- Swollen lymph glands
The severe WNV disease is also called the neuroinvasive disease, such as West Nile encephalitis or meningitis, or West Nile poliomyelitis.
About 1 in 150 people infected with the WNV have been estimated to develop a severe form of the disease.
The symptoms of the severe disease include:
- Headache
- High fever
- Neck stiffness
- Stupor
- Disorientation
- Coma
- Tremors
- Convulsions
- Muscle weakness
- Paralysis
- Myelitis
- Optic neuritis
- Rhombencephalitis
- Polyradiculitis
Rare extra neurological manifestations were also reported like myocarditis, pancreatitis, and fulminant hepatitis.
The severe illness can occur in patients of any age. However, people over the age of 60 years, immunocompromised, and patients with certain medical conditions like cancer, diabetes, hypertension, kidney disease, etc. are at a higher risk for severe illness if they are infected.
Diagnosis of West Nile Virus
Antibody Detection
The diagnosis of WVV infection is largely based on the detection of anti-WNV IgM antibodies in the serum and cerebrospinal fluid (CSF). The serological test is performed through the IgM-capture enzyme-linked immunosorbent assay (ELISA). The WNV-specific IgM antibodies are usually detectable 3 to 8 days after infection and may remain for 30 to 90 days, and hence may also reflect a past infection. Intrathecal IgM can indicate WNV infection in the central nervous system as the IgM antibodies do not cross the blood-brain barrier.
Plaque-reduction neutralization tests (PRNTs)
PRNTs are performed in the reference laboratories, including a few state public health laboratories and CDC. The test helps determine the specific flavivirus causing infection. PRNTs also confirm acute infections through demonstration of a fourfold or greater change in WNV-specific neutralizing antibody titer between acute and convalescent-phase serum samples collected 2 to 3 weeks apart.
Other tests
- Virus culture and detection of viral RNA (through the reverse transcriptase-polymerase chain reaction) can be done for the serum, CSF, and tissue specimens collected in the early stage of infection, and if positive, can confirm an infection.
- Immunohistochemistry (IHC) can also detect WNV antigens in formalin-fixed tissue.
- Viral culture, RT-PCR, and IHC can be done through state public health laboratories or CDC. However, the negative results of these tests do not rule out the possible infection of WNV.
- Diagnosis can also be made through brain biopsy from surgery and autopsy that detect pathological findings in fatal cases like inflammation of the brain and spinal cord with small hemorrhages, perivascular cuffing, and extensive neuronal degeneration.
- These are the result of WNV replication that cause injury, cytotoxic response, and inflammation.
Treatment of West Nile Virus
- There is no specific treatment for the WNV infection but, supportive treatment can be given to the patient.
- High doses of ribavirin and interferon-α 2b have shown efficacy against the WNV in vitro.
- However, sufficient clinical testing in vivo has yet to support its efficacies.
- Pain control for people with meningeal symptoms and antiemetic therapy and rehydration is required for patients with nausea and vomiting symptoms.
- Close monitoring should be done for patients with encephalitis for the development of elevated intracranial pressure and seizures.
- Ventilatory support should be given to patients in case of acute neuromuscular respiratory failure.
Prevention and Control of West Nile Virus
- No vaccines have been licensed for use against the WNV infection for humans to date.
- Although there are no FDA-approved vaccines for human use, there are effective and licensed vaccines for the treatment of horses against WNV infection.
- A number of vaccines are undergoing clinical trials for testing their efficacies.
- Primary preventions of the infection in humans include the use of mosquito repellents, wearing long sleeve clothing, long pants, and window screens in areas with a high prevalence of the disease.
- Mosquito breeding sites should be controlled to control the vector population that can reduce the transmission.
- Proper screening should be done to reduce transmission through blood transfusions and organ transplantations.
- Blood donations should not be done by patients with WNV infections for at least 4 months after their illness.
References
- Khan, S., & Chowdhury, P. (2021). Global emergence of West Nile virus: Threat & preparedness in special perspective to India. Indian Journal of Medical Research, 154(1), 36. https://doi.org/10.4103/ijmr.ijmr_642_19
- Rossi, S. L., Ross, T. M., & Evans, J. D. (2010). West Nile Virus. Clinics in Laboratory Medicine, 30(1), 47–65. https://doi.org/10.1016/j.cll.2009.10.006
- Colpitts, T. M., Conway, M. J., Montgomery, R. R., & Fikrig, E. (2012). West Nile Virus: Biology, Transmission, and Human Infection. Clinical Microbiology Reviews, 25(4), 635–648. https://doi.org/10.1128/cmr.00045-12
- Treatment & Prevention | West Nile Virus | CDC. (2018, December 10). Www.cdc.gov. https://www.cdc.gov/westnile/healthcareproviders/healthCareProviders-TreatmentPrevention.html
- West Nile virus – Diagnosis and treatment – Mayo Clinic. (n.d.). Www.mayoclinic.org. https://www.mayoclinic.org/diseases-conditions/west-nile-virus/diagnosis-treatment/drc-20350325
- Kemmerly, S. A. (2003). Diagnosis and Treatment of West Nile Infections. The Ochsner Journal, 5(3), 16–17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3111824/
- West Nile Virus. (2019). https://www.hopkinsmedicine.org/health/conditions-and-diseases/west-nile-virus
- West Nile virus | West Nile Virus | CDC. (2020, June 3). Www.cdc.gov. https://www.cdc.gov/westnile/index.html
- WHO. (2017, October 3). West Nile virus. Who.int; World Health Organization: WHO. https://www.who.int/news-room/fact-sheets/detail/west-nile-virus
