The most probable number (MPN) test is a statistical method test based on the random dispersion of microorganisms per volume in a given sample.
- In this method, measured volumes of water are added to a series of tubes containing a liquid indicator growth medium.
- The media receiving one or more indicator bacteria show growth and a characteristic color change. The color change is absent in those receiving only an inoculum of water without indicator bacteria.
- From the number and distribution of positive and negative reactions, the MPN of indicator organisms in the sample may be estimated by reference to statistical tables.
- MPN test is completed in three steps:
- Presumptive test
- Confirmed test
- Completed test
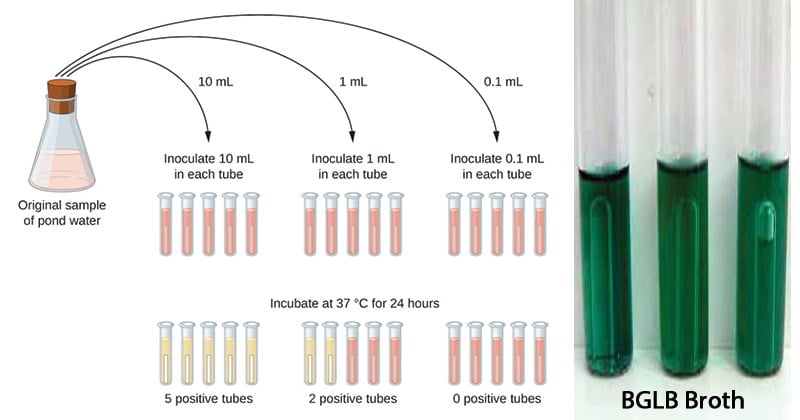
Image Source: Microbe Online and Scharlab.
Interesting Science Videos
Presumptive Test of MPN Test
This test, a specific enrichment procedure for coliform bacteria, is conducted in fermentation tubes filled with a selective growth medium (MacConkey lactose broth), which contains inverted Durham tubes for the detection of fermentation gas. A series of lactose broth tubes are inoculated with measured amounts of the water sample to be tested. The series of tubes may consist of three or four groups of three, five, or more tubes.
The main selective factors found in the medium are lactose, sometimes a surfactant such as Na-lauryl sulfate or Na-taurocholate (bile salt), and often a pH indicator dye for facilitating detection of acid production, such as bromcresol purple or brilliant green. The selective action of lactose occurs because many bacteria cannot ferment this sugar, whereas coliform bacteria and several other bacterial types can ferment it. The surfactant and dye do not inhibit coliform bacteria, whereas many other bacteria, such as the spore formers, are inhibited.
Confirmed Test of MPN Test
This test serves to confirm the presence of coliform bacteria when either a positive or doubtful presumptive test is obtained.
- A loopful of growth from such a presumptive tube is transferred into a tube of brilliant green lactose bile (BGLB) 2% broth (or other lactose broth) and incubated at 35°C for 48 hours. This is a selective medium for detecting coliform bacteria in water, dairy, and other food products. A selective agent in the medium is lactose. The broth tube also contains a Durham tube to detect gas production.
- A plate of LES Endo agar (or EMB agar) is streaked with a loopful of growth from a positive tube and incubated at 35°C for 18–24 hours. Typical coliform bacteria (E. coli and Enterobacter aerogenes) exhibit good growth on this medium and form red to black colonies with dark centers or a sheen. Salmonella typhi exhibits good growth but the colonies are colorless. S. aureus growth is inhibited altogether.
Completed Test of MPN Test
This test helps to further confirm doubtful and, if desired, positive confirmed test results. A typical coliform colony from an LES Endo agar plate is inoculated into a tube of brilliant green bile broth and on the surface of a nutrient agar slant. They are then incubated at 35°C for 24 hours. After 24 hours, the broth is checked for the production of gas, and a Gram stain is made from organisms on the nutrient agar slant. If the organism is a Gram-negative, non-spore-forming rod and produces gas in the lactose tube, then it is positive that coliforms are present in the water sample.
Objectives of MPN Test
- To enumerate the number of bacteria present in the drinking water by the MPN method.
- To identify the bacteria present in the drinking water sample.
Procedure of MPN Test
I. Presumptive Test
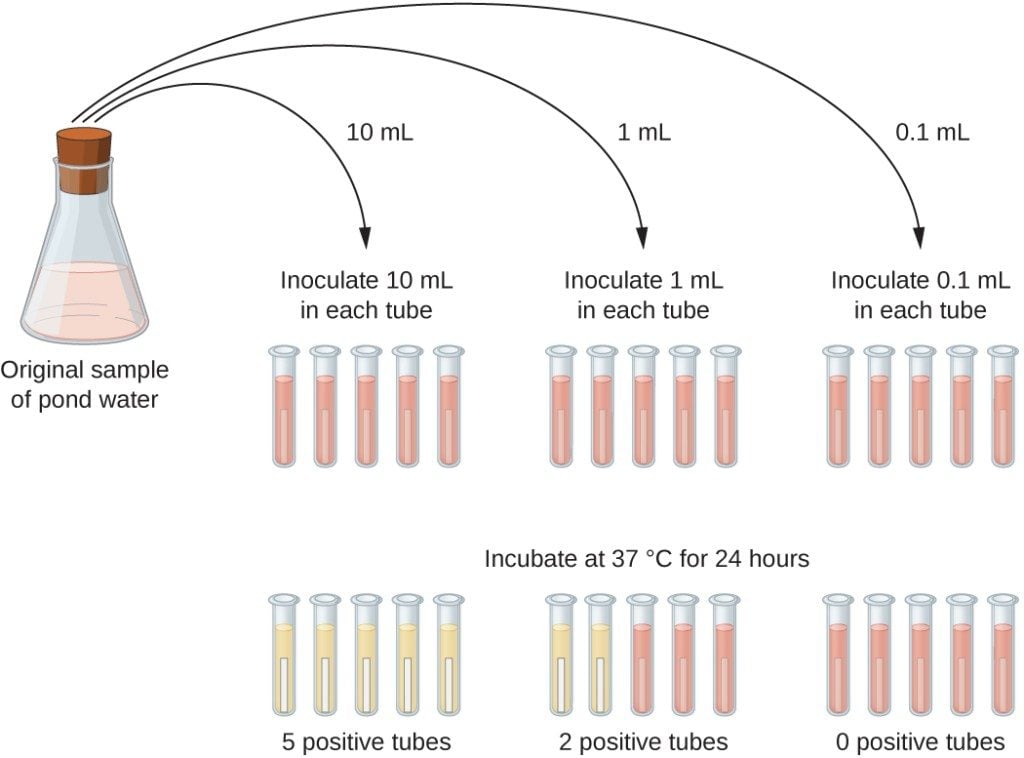
Image Source: Microbe Online
- Prepare MacConkey purple media of single and double strength in test tubes with Durham’s tube and autoclave it.
- Take three sets of test tubes containing five tubes in each set; one set with 10 ml of double strength (DS) other two containing 10 ml of single strength (SS) .
- Using sterile pipettes, transfer 10 ml of water to each of the DS broth tubes. Transfer 1 ml of water sample to each of 5 tubes of one set of SS broth and transfer 0.1 ml water to five tubes of remaining last set of SS broth tubes.
- Incubate the tubes at 37°C for 24 hours.
- After incubation, observe the gas production in Durham’s tube and the color change of the media.
- Record the number of positive results from each set and compare with the standard chart to give presumptive coliform count per 100 ml water sample.
II. Confirmed Test
Some microorganisms other than coliforms also produce acid and gas from lactose fermentation. In order to confirm the presence of coliform, a confirmatory test is done. For this, a loopful of suspension from a positive tube is inoculated into a 3 ml lactose-broth or brilliant green lactose fermentation tube and to an agar plate (EMB agar or Endo Agar) or slant.

A. Inoculation of the lactose-broth
- Incubate the inoculated lactose-broth fermentation tubes at 37°C and inspect gas formation after 24 ± 2 hours.
- If no gas production is seen, further incubate up to a maximum of 48 ±3 hours to check gas production.
B. Inoculation in media slants
- Take a loopful of suspension from a positive tube and inoculate it on the agar surface.
- The agar slants should be incubated at 37°C for 24± 2 hours.
- Colonies must be examined macroscopically.
III. Completed Test
- Transform a typical coliform colony from the agar plate into a tube of brilliant green bile broth with placed Durham’s tube and on the surface of a nutrient agar slant.
- Incubate at 35°C for 24 hours.
- After 24 hours, check the broth for the production of gas, and perform Gram staining for organisms on the nutrient agar slant.
Results of MPN Test
A. Presumptive Test
Positive: The formation of 10% gas or more in the Durham tube within 24 to 48 hours, together with turbidity in the growth medium and the color change in the medium constitutes a positive presumptive test for coliform bacteria, and hence for the possibility of fecal pollution.
- The test is presumptive only because under these conditions several other types of bacteria can produce similar results.
Negative: No growth or formation of gas in Durham’s tube.
B. Confirmed Test
Positive: Formation of gas in lactose broth and the demonstration of a coliform-like colony on the EMB agar indicate the presence of a member of the coliform group in the sample examined.
Coliforms produce colonies with a greenish metallic sheen which differentiates them from non-coliform colonies (show no sheen). The presence of typical colonies at high temperatures (44.5 ±0.2) indicates the presence of thermotolerant E.coli.
Negative: The absence of gas formation in lactose broth or the failure to demonstrate coliform-like colonies on the EMB agar.
Completed Test
Positive: The presence of gas in the brilliant green bile broth tube and Gram-negative, non-spore-forming rods on NA slant constitutes a positive completed test for the presence of coliform bacteria, which, in turn, infers possible contamination of the water sample with fecal matter.
Negative: Absence of growth and gas formation in the broth. Absence of gram-negative, non-sporing rods on Gram staining.
Uses of MPN Test
- It is commonly used in estimating microbial populations in soils, waters, agricultural products.
- The technique is particularly useful with samples that contain particulate material that interferes with plate count enumeration methods.
- It has also been suggested as a consideration for an alternate method to trend environmental monitoring studies.
- It is also useful for counting bacteria that reluctantly form colonies on agar plates or membrane filters but grow readily in liquid media.
Advantages of MPN Test
- Ease of interpretation, either by observation or gas emission
- Sample toxins are diluted
- Effective method of analyzing highly turbid samples such as sediments, sludge, mud, etc.
- Permits samples that cannot be analyzed by membrane filtration.
Limitations of MPN Test
- Poor accuracy and precision associated with MPN count usually mean that the method is one of last resort — to be considered only when other counting methods are inappropriate.
- Laborious and expensive in terms of materials, glassware, and incubator space.
- It has relatively a large margin of error.
References
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA
- http://microbiol.org/wp-content/uploads/2010/12/Sutton.jvt_.16.3.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC92996/
- https://www.slideshare.net/prakashtu/bacteriological-analysis-of-drinking-water-by-mpn-method
- https://microbeonline.com/probable-number-mpn-test-principle-procedure-results/
- http://fire.biol.wwu.edu/brodham/biol346_S07/labman_week9b.pdf

Most probable number (MPN) is a method of testing water quality by estimating the number of microorganisms present. This method is commonly used to test for the presence of bacteria, but can also be used to test for other microorganisms, such as viruses and protozoa. https://www.vipananlab.com/
Very good explanation in simple language.
I live near water that has 2218 per, according to … Been advised likely somewhere along the pit a septic is the culprit. My plan is to sample and test at different places to confirm to hone in on the source. This will require a DIY test that can give me a measurable result. Does anybody know if such a kit exists? My email is randy57@twc.com