Interesting Science Videos
Structure of Varicella Zoster Virus
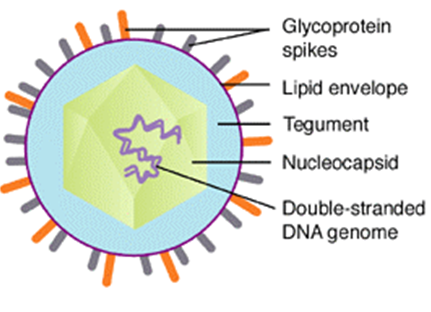
Image Source: Creative Diagnostics
- Varicella-zoster virus (VZV) is an alphaherpesvirus that is in the same subfamily as herpes simplex virus (HSV) 1 and 2.
- VZV is a member of varicellovirus genus.
- The virion is spherical in shape with icosahedral symmetry measuring 159-200nm.
- The icosahedral protein capsid with average diameter 100 nm consists of 162 hollow hexagonal and pentagonal capsomeres with an electron-dense core containing the double-stranded DNA genome with 125-240 kbp nucleotides together forming the nucleocapsid.
- The nucleocapsid is surrounded by an envelope which is lipoprotein in nature.
- Lipid part is derived from the nuclear membrane of the infected host cell.
- The enveloped particle is pleomorphic to spherical, and 180 to 200 nm in diameter.
- Projecting from the trilaminar lipid host-derived envelope are spikes of viral glycoproteins, 8nm long, which bind to specific host receptor and mediate virus entry.
- gB, gE and gH are proteins abundantly found and mediate primary attachment to host cell surface, glycosaminoglycan and fusion.
- In mature virus particles, outside the capsid is an amorphous proteinaceous layer, the tegument, surrounded by a lipid envelope derived from host cell membranes.
- The tegument consists of enzymes such as VP16 which is responsible for subverting cellular proteins and enzymes to involve in viral nucleic acid replication and VHS (Virion Host Shutoff ) protein which shut off the host cell protein synthesis in the cytoplasm.
Genome of Varicella Zoster Virus
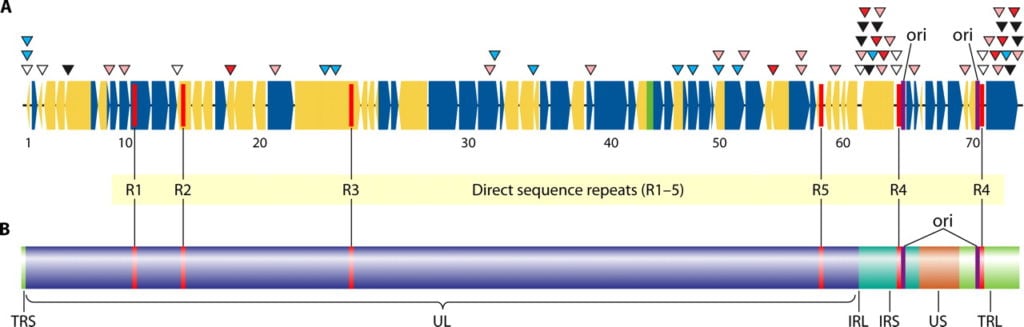
Image Source: DOI: 10.1128/CMR.00031-09
- The virus contains a double-stranded DNA genome and is linear measuring 125 kbp in length.
- The genome consists of a unique long region (UL) bounded by terminal long (TRL) and internal long (IRL) repeats, and a unique short region (US) bounded by internal short (IRS), and terminal short (TRS) repeats.
- The varicella-zoster virus (VZV) genome contains at least 70 genes.
- VZV encodes at least 3 immediate-early (IE) proteins that are located in the tegument of virions and regulate virus transcription.
- The VZV genome contains about 41 “core genes” that are conserved with each of the three subfamilies of herpesviruses, alphaherpesvirus, betaherpesvirus, and gammaherpesvirus.
- Core genes include IE4, the VZV DNA polymerase, helicase-primase components, single-stranded DNA-binding protein, ribonucleotide reductase, uracil-DNA glycosylase, dUTPase, DNase, ORF47 protein kinase, major capsid protein, protease, assembly protein, several tegument proteins, gB, gH, gL, gM, and gN.
Epidemiology and transmission of Varicella Zoster Virus
- Varicella occurs throughout the year in temperate regions, but the incidence typically peaks in the months of March through May.
- According to national seroprevalence data from the pre-vaccine era, greater than 95 percent of persons in the United States acquired varicella before 20 years of age, and fewer than 2 percent of adults were susceptible to infection.
- Prior to 1995, the Centers for Disease Control and Prevention (CDC) estimated the yearly incidence of chickenpox in the United States at approximately four million cases, with nearly 11,000 admissions and 100 deaths.
- Varicella is one of the classic diseases of childhood, with the highest prevalence occurring in the 4 – 10 years old age group.
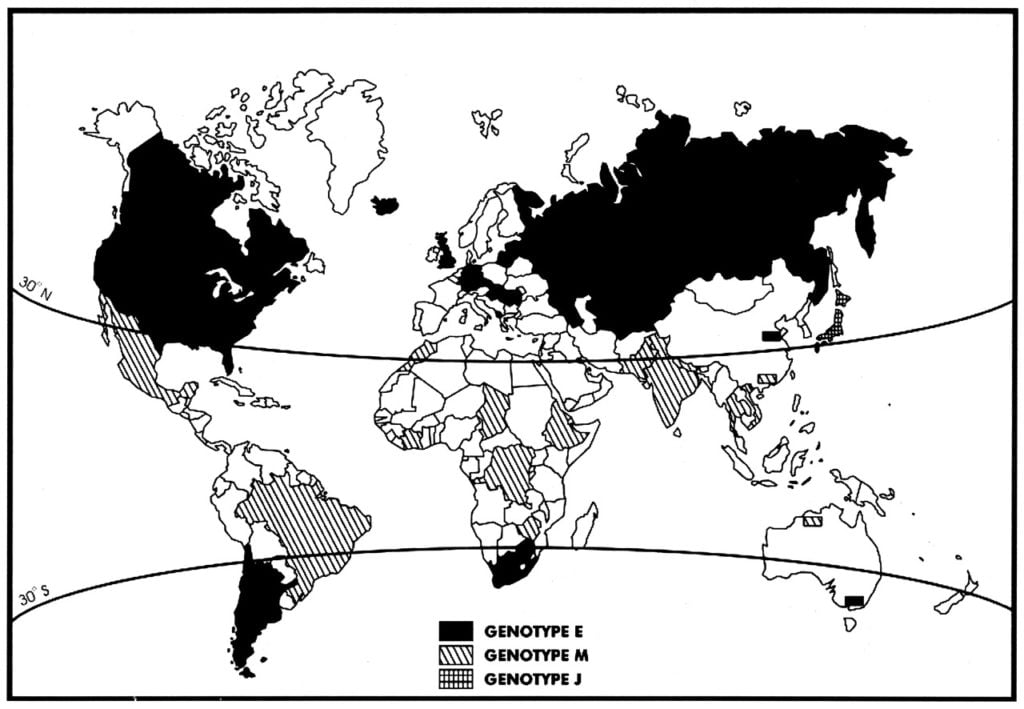
Figure: Global distribution of the three major genotypes.
Image Source: DOI: 10.1128/JVI.78.15.8349-8358.2004
- Varicella is highly communicable, with an attack rate of 90% in close contacts.
- Chickenpox is a very contagious disease caused by the varicella-zoster virus and spreads easily from people mainly by touching or breathing in the virus particles that come from chickenpox blisters, and possibly through tiny droplets from infected people that get into the air after they breathe or talk.
Replication of Varicella Zoster Virus
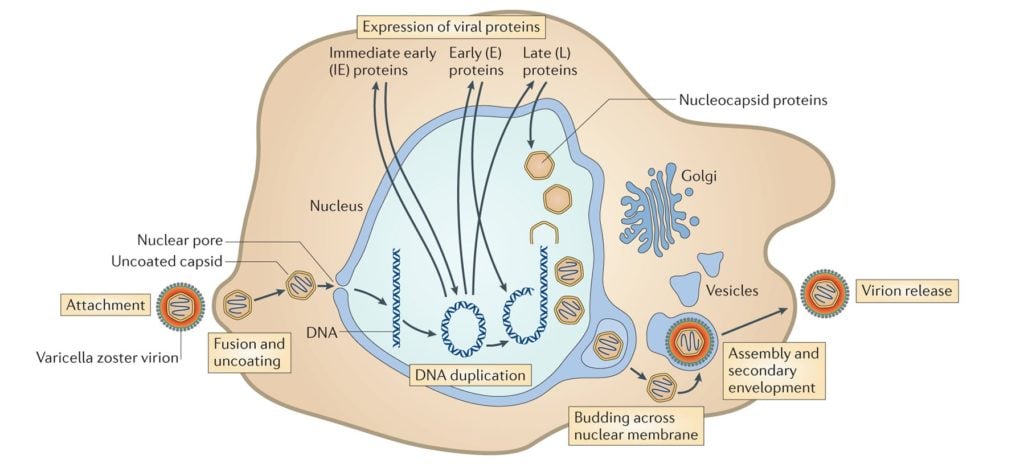
Image Source: DOI: 10.1038/nrmicro3215
- Attachment of the viral glycoproteins to host receptors mediates endocytosis of the virus into the host cell.
- Fusion with the plasma membrane to release the core and the tegument proteins into the host cytoplasm.
- The incoming viral capsids are subsequently propelled to nucleopore for entry in the nucleus where it gets disintegrated and only DNA is released into the nucleus.
- The viral genome is uncoated for viral transcription and replication in the nucleoplasm.
- There are two main phases of transcription–early, which takes place prior to genome replication, and late, which takes place upon replicated genomes in virus replication compartments formed in the infected cell nucleus.
- Three distinct classes of mRNAs are made: Alpha, Beta, and Gamma which are regulated in a coordinated, cascade fashion.
- The Alpha or IE (Immediate-early) genes contain the major transcriptional regulatory proteins and their production is required for the transcription of the Beta and Gamma gene classes.
- The Beta proteins include the enzymes that are required for replication of the viral genome: a DNA polymerase, a single-strand DNA-binding protein, a primosome or helicase-primase, an origin-binding protein, and a set of enzymes involved in DNA repair and in deoxynucleotide metabolism.
- Viral DNA synthesis begins shortly after the appearance of the Beta proteins and the temporal program of viral gene expression ends with the appearance of the Gamma or late proteins, which constitute the structural proteins of the virus.
- The linear genome circularizes shortly after infection of susceptible host cells and then enters a rolling circle mode of DNA replication generating branched concatemeric DNA, which is then cleaved to release linear ds DNA.
- Viral transcription and DNA replication occur in the nucleus; the particle assembles and exits from epithelial cells in the skin causing a primary infection.
- The virion acquires its envelope by budding through the nuclear membrane.
Pathogenesis of Varicella Zoster Virus
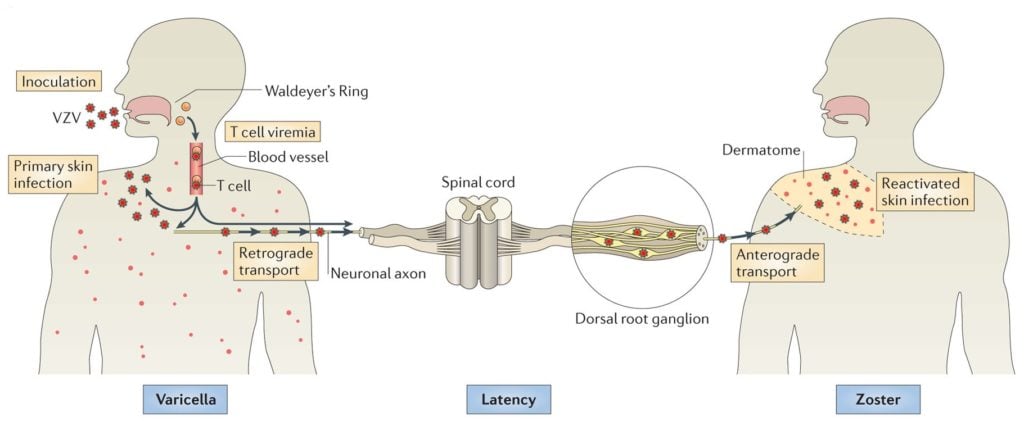
Image Source: DOI: 10.1038/nrmicro3215
- The portal of entry of virus is via the inhalation of respiratory droplets through the respiratory tract or also via the contact with infected persons.
- The virus infects the upper respiratory tract.
- The virus replicates and proliferates in regional lymph nodes of the URT.
- This follows the primary viremia in the bloodstream.
- From the bloodstream, the virus localizes in the skin, mucous membrane and other organs particularly liver and spleen which results in the secondary viremic stage.
- This stage is manifested by the appearance of characteristics rashes composed of macules which rapidly develop into fluid-filled vesicles.
- Within three days, the fluid becomes cloudy with the influx of leukocytes, fibrin, and interferon.
- The pustules then dry up, scabs form and they desquamate.
- Lesions and vesicles are present in all stages then after.
- The virus infected host cells are cleared by T-Cell-mediated immune mechanism and Antibody-Dependent Cell-mediated Cytotoxicity (ADCC).
- The virus may also enter nerve endings and transport to dorsal root ganglia where it remains dormant in sensory nerve ganglia, dorsal root.
- After infection, the virus remains latent in cranial-nerve and dorsal-root ganglia, with reactivation causing dermatomal, or in the immunocompromised host, disseminated zoster (“shingles”).
Clinical Presentation of Varicella Zoster Virus
- Varicella-zoster virus (VZV) infection causes two clinically distinct forms of the disease.
Varicella
- Primary infection with VZV results in varicella (chickenpox), characterized by vesicular lesions in different stages of development on the face, trunk, and extremities.
- The incubation period for varicella is 14 to 16 days after exposure to varicella or a herpes zoster rash, with a range of 10 to 21 days.
- A mild prodrome of fever and malaise may occur 1 to 2 days before rash onset, particularly in adults and in children, the rash is often the first sign of disease.
- The rash is generalized and pruritic (itchy).
- It progresses rapidly from macules to papules to vesicular lesions before crusting.
- The rash usually appears first on the head, chest, and back then spreads to the rest of the body.
- In case of children, the signs and symptoms are generally mild with an itchy rash, malaise, and temperature up to 102°F for 2 to 3 days.
- Infants, adults, and immunocompromised people are at risk for more severe disease and have a higher incidence of complications.
- Severe complications caused by varicella include cerebellar ataxia, encephalitis, viral pneumonia, and hemorrhagic conditions.
- Other complications include septicemia, toxic shock syndrome, necrotizing fasciitis, osteomyelitis, bacterial pneumonia, and septic arthritis.
- Several discreet neurologic syndromes have been attributed to VZV infection.
- Acute cerebellar ataxia is a complication of primary varicella infection and does not occur with viral reactivation.
- Patients with acute cerebellar ataxia develop acute gait ataxia, nystagmus, vomiting, tremor, and headache, although usually have intact cognition.
- Symptoms typically begin in the 10 days following cutaneous eruption; however, rarely there may be up to three-week latency between rash and onset of cerebellar symptoms.
- Full recovery generally occurs within weeks to months.
- Another neurologic syndrome suggestive of VZV is CNS vasculitis of either large or small blood vessels.
Herpes- zoster
- Herpes zoster, also known as shingles, results from reactivation of endogenous latent VZV infection within the sensory ganglia.
- People with herpes zoster most commonly have a rash in one or two adjacent dermatomes (localized zoster).
- The rash most commonly appears on the trunk along a thoracic dermatome.
- Less commonly, the rash can be more widespread and affect three or more dermatomes.
- The rash is usually painful, itchy or tingly.
- These symptoms may precede rash onset by days to weeks and some people may also have a headache, photophobia (sensitivity to bright light), and malaise in the prodromal phase.
- The rash develops into clusters of vesicles.
- New vesicles continue to form over three to five days and progressively dry and crust over and they usually heal in two to four weeks.
- There may be permanent pigmentation changes and scarring on the skin.
- Complications in herpes zoster infection include Postherpetic neuralgia (PHN), ophthalmic involvement with acute or chronic ocular sequelae (herpes zoster ophthalmicus), bacterial superinfection of the lesions, cranial and peripheral nerve palsies and visceral involvement, such as meningoencephalitis, pneumonitis, hepatitis, and acute retinal necrosis.
Laboratory diagnosis of Varicella Zoster Virus
Microscopy
- Direct detection methods include:
- Cytology– smears of scrapings of the base of the lesions will reveal characteristic multinucleate giant cells, also known as Tzanck cells.
- Electron microscopy– herpesvirus particles can be seen in fluid taken from the early vesicles of either varicella or zoster.
- Cytology and electron microscopy, however, cannot differentiate between HSV and VZV.
- Immunofluorescence cytology– smears of the base of lesions can be examined by immunofluorescence cytology, as in the case for HSV.
- This technique is more sensitive than EM but is more labor intensive and requires greater technical expertise.
Molecular Methods
- PCR assays for VZV are available and have been reported to be of use in the diagnosis of VZV meningoencephalitis from CSF specimens.
Virus isolation
- Virus culture in human fibroblasts are used in most laboratories.
- Vesicle fluid and scrapings from the base of fresh lesions are the most suitable specimens.
- The CPE produced by VZV reveals typical ballooning and fusion.
- Immunofluorescence of the cell sheet by monoclonal antibodies is the method of choice for identification.
- Virus isolation for VZV is rarely carried out because of the long length of time required for a result to be available.
- Serology
- Serological diagnosis of primary varicella infection can be reliably carried out using paired acute and convalescent sera.
- Detection of VZV specific IgM can be determined by IF (Immunofluorescence) and capture RIA or EIA.
- Other tests include Complement fixation test (CFT) and latex agglutination.
Treatment of Varicella Zoster Virus
- VZV is sensitive to acyclovir and is a licensed treatment procedure for chickenpox.
- Other antiviral medications that may also work against chickenpox include valacyclovir and famciclovir.
- Convalescent pooled serum containing varicella zoster immunoglobulin (VZIG) gives passive protection in immunocompromised children exposed to infection, however, it cannot be considered as an effective treatment.
Prevention and control of Varicella Zoster Virus
- Dr. Michiaki Takahashi developed the first chickenpox vaccine.
- Preventing varicella in healthcare settings is done by following standard precautions plus airborne precautions (negative air-flow rooms) and contact precautions until lesions are dry and crusted.
- The best preventive measure for chickenpox is the chickenpox vaccine.
- The vaccine is a live attenuated vaccine and administered subcutaneously.
- Two doses of the vaccine are about 90% effective at preventing chickenpox.

this was very informative and well written and organized. thank you