The Urease test is a biochemical test that detects the alkaline fermentation of urine (urea) with the resultant production of ammonia by microorganisms.
- The fermentation of urea occurs in the presence of the enzyme ‘urease’, resulting in two molecules of ammonia and carbon dioxide.
- Urease activity is one of the important characteristics for the identification of Proteus species and allows for Proteus to be distinguished from non-lactose-fermenting members of the Enterobacteriaceae.
- Christensen developed the test in 1946 for the differentiation of enteric bacilli. The urea agar base used for the testing of urease activity is named Christensen Urea Agar after him.
- During the test, the organisms utilize urea as the sole source of nitrogen, producing a sufficient amount of ammonia to overcome the buffering capacity of the medium.
- The change in color of the medium as a result of the change in pH is indicative of the test result.
Objectives of Urease Test
- To test the ability of an organism to produce the enzyme urease that hydrolyses urea.
- To differentiate urease-positive Proteus from other Enterobacteriaceae.
Microorganisms Tested
The urea test is part of the battery of tests to identify the following:
- Gram-negative enteric pathogens, including Yersinia spp.
- Fastidious Gram-negative rods—Brucella, Helicobacter pylori, and Pasteurella.
- Gram-positive rods—Corynebacterium and Rhodococcus spp.
- Yeasts—Cryptococcus spp.
Directly, this test is performed as a rapid test on gastric biopsy samples to detect the presence of H. pylori.
Interesting Science Videos
Principle of Urease Test
Urea medium, whether a broth or agar, contains urea and the phenol red as a pH indicator. Many organisms, especially those that cause urinary tract infections, produce the urease enzyme, which catalyzes the splitting of urea in the presence of water to release two molecules of ammonia and carbon dioxide. The ammonia combines with the carbon dioxide and water to form ammonium carbonate, which turns the medium alkaline, turning the indicator from its original orange-yellow color to bright pink. This test is performed as part of the identification of several genera and species of the Enterobacteriaceae family, including Klebsiella, Proteus, and some Citrobacter and Yersinia species, as well as some Corynebacterium species. The test is also useful to identify Cryptococcus, Brucella, Helicobacter pylori, and many other bacteria that produce the urease enzyme. Disks are available that combine urea and phenylalanine deaminase (PDA), allowing a one-disk test to identify Proteus, Providencia, and Morganella and to separate them from Klebsiella and Yersinia enterocolitica. The disk reactions are rapid and sensitive and allow for the rapid detection of agents of serious infections, g., Brucella, and Cryptococcus.
Media, Reagents, and Supplies Used
Media Used
- Both urea agar slants and broth media can be used for the detection of urease production. Agar media includes the Urea Agar Base (Christensen agar), and the broth includes the urea broth.
- Besides, rapid test kits are also available for the detection of urease activity.
- The composition of the urea agar base is given below:
| S.N | Ingredients | Gram/liter |
| 1. | Dextrose | 1.0 |
| 2. | Peptic digest of animal tissue | 1.5 |
| 3. | Sodium chloride | 5.0 |
| 4. | Monopotassium phosphate | 2.0 |
| 5. | Phenol red | 0.012 |
| 6. | Agar | 15.0 |
| Final pH at 25°C: 6.8 ±0.2 |
Supplies Used
- Sterile wooden sticks or loops
- Saline or water in a small plastic tube for the disk test
- Incubator at 35°C and 30°C
Procedure of Urease Test
A. Preparation of media
- About 24.52 grams of the dehydrated medium is dissolved in 950 ml distilled water in a beaker.
- The solution is heated to bring it to a boil in order to dissolve the medium completely.
- The prepared suspension is sterilized by autoclaving at 15 lbs pressure, 121°C for 15 minutes.
- The beaker is taken out following the autoclaving and cooled to 50°C. To the beaker, 50 ml of sterile 40% urea solution is added and mixed well.
- The medium is dispensed into tubes and set in a position to obtain agar slants.
B. Urease Activity
- A loopful of a well-isolated colony is taken with an inoculating loop and inoculated on the agar slants. The inoculation should be done on just the slant, and the butt shouldn’t be stabbed.
- The tubes are then incubated with loosened caps at 35 to 37°C. For non-fermenters, the tubes are incubated at 30°C.
- The tubes are observed for the development of pink color for as long as 7 days.
- If no growth is seen on the slant, further inoculation with heavy inoculum should be done.
C. Rapid Urease Test
- Rapid urease test is also called the CLO test (Campylobacter-like organism) and is used for the rapid identification of Helicobacter pylori.
- For the rapid urease test, a biopsy is taken of the mucosal layer of the antrum of the stomach and placed on the urea broth with phenol red indicator.
- The tube is then observed for the change in color from yellow to pink.
Result Interpretation of Urease Test
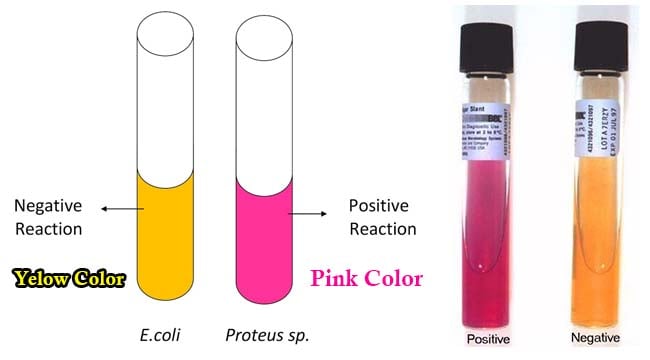
- A positive test is demonstrated by an intense magenta to bright pink color in 15 min to 24 hours.
- A negative test shows no color change.
Control organisms
- Positive test: Proteus mirabilis.
- Negative test: Escherichia coli.
Uses of Urease Test
- Urease test is used to identify organisms that are capable of hydrolyzing urea to produce ammonia and carbon dioxide.
- The test is particularly used for the presumptive identification of Proteus species for other members of the Enterobacteriaceae family.
- The test also differentiates Proteus from the non-lactose-fermenting bacteria.
- This test is performed as a rapid test on gastric biopsy samples to detect the presence of H. pylori.
Limitations of Urease Test
- Some organisms should rapid result as they rapidly split urea (Brucella and H. pylori), while others might react slowly.
- When performing overnight tests from a medium that contains peptone, the alkaline reaction may be due not to urease but to hydrolysis of peptone.
- Urea is light sensitive and might undergo autohydrolysis. The medium thus must be stored at 2 to 8°C in the dark.
- The test is less sensitive if the medium is not buffered.
References and Sources
- Biochemical Tests for the Identification of Aerobic Bacteria. (2016). Clinical Microbiology Procedures Handbook, 3.17.1.1–3.17.48.3.DOI:10.1128/9781555818814.ch3.17.1
- Urea Agar Base (Christensen). M112S. HiMedia Laboratories.
- Benita Brink. 2010. Urease test protocol.
- 5% – https://microbiologyinfo.com/urease-test-principle-media-procedure-and-result/
- 4% – https://microbeonline.com/urease-test-principle-procedure-interpretation-and-urease-positive-organsims/
- 3% – https://medicalstudyzone.com/urease-test-for-urea/
- 3% – https://asm.org/getattachment/ac4fe214-106d-407c-b6c6-e3bb49ac6ffb/urease-test-protocol-3223.pdf
- 2% – https://asm.org/Protocols/Urease-Test
- 1% – https://www.sciencedirect.com/science/article/pii/B9780128119426000157
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4008738/
- 1% – https://www.austincc.edu/microbugz/urease_test.php
- 1% – https://microbenotes.com/acetate-utilization-test-principle-procedure-and-result-interpretation/
- 1% – https://blairgastro.com/clo-testing/
- 1% – http://himedialabs.com/TD/M112.pdf
- 1% – http://europepmc.org/articles/PMC4008738
- <1% – https://www.ncbi.nlm.nih.gov/pubmed/11375581/
- <1% – https://catalog.hardydiagnostics.com/cp_prod/content/hugo/UreaPDADisks.htm
- <1% – http://www.ijabmr.org/article.asp?issn=2229-516X;year=2016;volume=6;issue=1;spage=18;epage=22;aulast=Roy
- <1% – http://urinarytractinfectioncauses.net/organisms-that-cause-urinary-tract-infection/

Videos please
Sure, soon
We ‘ve already seen the colours.
Colour yellow is -ne
Colour pink is +ve
So what known as this disease …………….?
Also used for rapid detection of Corynebacterium urealyticum, a pathogen of the urinary tract, seen in individuals who have had a transplant. I’ve also seen it used as a rapid test to rule in a possible Brucella sp when suspected on the basis of gram and colony characteristics.