Interesting Science Videos
Classification of Torovirus
Group IV: ssRNA positive strand virus
Order: Nidovirales
Family: Coronaviridae
Subfamily: Toronavirinae
Genus: Torovirus
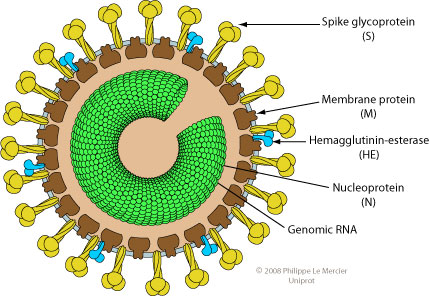
Structure of Torovirus
- Torovirusis a genus of viruses in the order Nidovirales, in the family Coronaviridae, in the subfamily Torovirinae.
- Toroviruses are pleomorphic, enveloped particles, 120–140 nm in diameter.
- The virus particle has surface spikes proteins that are club-shaped 15 to 20 nm in length and are evenly dispersed over the surface.
- A nucleocapsidis doughnut-shaped with helical symmetry.
- The tubular nucleocapsid is bend into an open torus hence the name “torovirus”.
- The molecular weights of the nucleocapsid protein is 19 kDa.
- These are enveloped viruses with non-segmented and positive-sense (single stranded) RNA genome of 20 to 25 kilobases.
- Virions contain the glycoproteins spikes (S), membrane protein (M), a core nucleoprotein (N) and hemagglutinin-esterase(HE)
- Toroviruses have a buoyant density of 1.14–1.18 g/cm3 in sucrose.
- Virus infectivity is stable between pH 2.5 and 9.7 but rapidly inactivated by heat, organic solvents, and irradiation.
Genome of Torovirus
- The genome of torovirus is monopartite and linear single-stranded, positive-sense RNA of about 20 kb.
- The genome is capped at 5’end and polyadenylated at 3’end.
- The virion RNA is infectious and serves as both the genome and viral messenger RNA.
- Genomic RNA encodes for ORF1a, as for ORF1b, it is translated by ribosomal frameshifting.
- The first two reading frames ORF1a and ORF1b from the 5’ end are translated from genomic RNA and constitute the viral replicase enzymes.
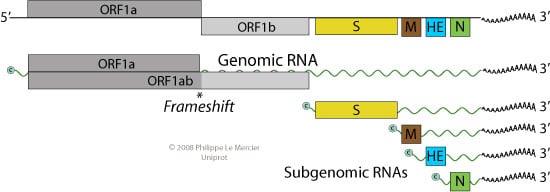
- Resulting proteins pp1a and pp1ab are processed into the viral polymerase (RdRp) and other non-structural proteins involved in RNA synthesis.
- Structural proteins are expressed as subgenomic RNAs.
- Each RNA (genomic and subgenomic) is translated to yield only the protein encoded by the 5′-most ORF.
Epidemiology of Torovirus
- The geographic distribution of torovirus in human and animals is worldwide, with the virus identified in countries such as Brazil, Belgium, Canada, Costa Rica, France, Germany, Great Britian, Hungary, India, The Netherlands, New Zealand, South Africa, the United States, and most recently Austria.
- Toroviruses have been detected in stools of children and adults with diarrhea in developed countries.
- The virus, originally designated Breda virus, was first isolated during an outbreak of severe neonatal gastroenteritis with 56.5% morbidity and 8.7% mortality in cattle from dairy farms round the township Breda, Iowa, and duly identified as the etiological agent.
Replication of Torovirus
- Attachment of the viral S protein or HE protein to host receptors mediates endocytosis of the virus into the host cell. Endocytosis can occur via clathrin-mediated endocytosis, caveolin-mediated endocytosis or clathrin- and caveolin-independent endocytosis.
- Synthesis and proteolytic cleavage of the replicase polyprotein.
- Fusion of virus membrane with the endosomal membrane, releasing ssRNA(+) genome into the cytoplasm.
- Synthesis and proteolytic cleavage of the replicase polyprotein occurs.
- Replication occurs in viral factories where a dsRNA genome is synthesized from the genomic ssRNA(+).
- The replication occurs in membranous invaginations of the REG, possibly to avoid dsRNA intermediate detection.
- The dsRNA genome is transcribed/replicated thereby providing viral mRNAs/new ssRNA(+) genomes.
- Synthesis of structural proteins encoded by subgenomic mRNAs.
- Release of new virion by budding at endoplasmic reticulum (ER) or Golgi apparatus which implies that the viral particle are exported by cellular exocytosis.
Pathogenesis of Torovirus
In calf
- Transmission is probably via the oral/nasal route through contact with contaminated feces or nasopharyngeal secretions.
- Breda virus, the bovine torovirus, is pathogenic for newborn gnotobiotic and nonimmune conventional calves.
- These animals develop watery diarrhea lasting for 4–5 days, with virus shedding for at least several days thereafter.
- Histologic lesions include necrosis of enterocytes with subsequent villus contraction (atrophy) from mid-jejunum to distal ileum, in addition to enterocyte necrosis in the large intestine.
- Epithelial cells lining both the intestinal crypts and villi are infected.
- The germinal centers of the Peyer’s patches become depleted of lymphocytes.
- There is necrosis of dome epithelial cells, including M cells.
In human
- Human torovirus (HToV) is a causal agent of human enteric infections and a source of nosocomial infection in immunocompromised individuals.
- Human torovirus is associated with gastroenteritis leading to diarrhea (acute or chronic form).
- First seroconversion occurs in many patients shedding HToV, whereby the affected individual develops a specific antibody response as measured using an HI test with purified HToV.
Clinical Manifestations of Torovirus
In calf
- Toroviruses cause diarrhea in calves by infecting villous and crypt enterocytes of the mid-jejunum, ileum, colon and cecum and inducing villous atrophy and necrosis of the crypts.
- Clinical signs are pyrexia, diarrhoea, dehydration, lethargy and depression in calves as well adults.
- In calves, it may lead to anorexia, mucoid faeces and neurological signs like generalised weakness, paralysis, inability to stand along with trembling and sudden death.
In human
- The clinical forms caused by human torovirus include acute diarrhea, persistent (chronic) diarrhea, necrotizing enterocolitis in children.
Lab Diagnosis of Torovirus
In calves
- Using immunofluorescence, Breda virus antigen can be detected in epithelial cells of the small intestine.
- Torovirusparticles also can be directly visualized in feces or intestinal contents, using electron microscopy.
- Immunological tests include Immuno-electron Microscopy (IEM) using hyperimmune antiserum is preferred for definitive identification of torovirus–antibody complexes,
- Haemagglutination Inhibition (HI), Enzyme Linked Immunosorbent Assay (ELISA) and Southern blot for antibody detection.
- The molecular diagnostic assays for Torovirus infection are reverse transcription-polymerase chain reaction (RT-PCR) using torovirus-specific primers, one step multiplex reverse transcription-PCR, nested-RT-PCR targeting the Nucleocapsid (N) gene and SYBR Green real-time RT-PCR.
- Among bovine torovirus, only Aichi/2004 strain has been isolated human rectal adenocarcinoma cell line (HRT-18).
In Human
- Detection by electron microscopy of virus particles with characteristic torovirus
- Detection of viral antigen or RNA by ELISA or RT-PCR, respectively, using Berne or Breda virus-specific reagents.
- Berne virus neutralizing antibodies are also detected in human sera.
- Antibody detection by Haemagglutination Inhibition(HI) using purified HToV.
Treatment of Torovirus
- There is no specific treatment.
- However, animals suffering from torovirus infection can be treated symptomatically by fluid therapy to prevent dehydration.
- Antibiotics may be used to check and prevent the secondary bacterial infections.
- A specially formulated ORS with less sodium, more potassium and glucose and the addition of magnesium, copper and zinc, as well as continuous feeding with calorie-dense foods, are recommended for malnourished patients.
Prevention and control of Torovirus
- No vaccine is available for torovirus.
- Colostrum containing antibodies against BToV can be used as prophylaxis.
- The affected animals must be isolated from apparently healthy animals.
- Proper personal hygiene like wearing of gloves, boots and gowns are necessary in the farms during the handling of affected animals to avoid chances of cross contamination.
- Faecal matter and bedding material should not be spread on pastures.
- Floor and walls of animal shed should be properly disinfected using suitable detergents and disinfectants.
- Empowering public health and animal surveillance systems.
- Good management practices including of hygiene and sanitary conditions along with strict biosecurity measures need to be maintained to combat toroviruses.
