Tollens’ test is a chemical test used to differentiate reducing sugars from non-reducing sugars. This test is also called the silver mirror test based on the end product of this test.
Objectives of Tollens’ test
- To distinguish reducing sugars from non-reducing sugars.
- To detect the presence of aldehyde containing carbohydrates and differentiate them from ketone containing carbohydrates.
Interesting Science Videos
Principle of Tollens’ test
- The Tollen’s reagent is the alkaline solution of silver nitrate (AgNO3) mixed with liquid ammonia (NH3), which results in the formation of a complex.
- The aqueous solution of silver nitrate forms a silver aqua complex where the water acts as a ligand.
- The aqua complexes are then converted into silver oxides (Ag2O) by the action of hydroxide ions.
- Silver oxide forms a brown precipitate, which is then dissolved by aqueous ammonia resulting in the formation of the [Ag(NH3)2]+ complex.
- This complex is the primary component of the Tollen’s reagent and is a strong oxidizing agent.
- The complex then oxidizes the aldehyde group present in some sugars to form a carboxylic acid.
- At the same time, the silver ions present in the reagent are reduced to metallic silver.
- The reduction of silver ions into metallic silver results in the formation of a silver mirror on the bottom and sides of the test tube.
- However, an α-hydroxy ketone gives a positive Tollen’s test as the Tollen’s reagent oxidizes the α-hydroxy ketone into an aldehyde.
Reaction
2AgNO3 + 2NaOH → Ag2O (brown ppt) + 2NaNO3 + H2O
Ag2O (brown ppt) + 4NH3 + 2NaNO3 + H2O → 2[Ag(NH3)2]NO3 + 2NaOH
Glucose + 2[Ag(NH3)2]NO3 + H2O → 2 Ag(silver mirror) + 4 NH3 + Gluconic acid + 2 H+
Requirements
Reagent
- Tollen’s reagent: Add 50 ml of 0.1 M AgNO3 to a beaker and to this, add 25 ml of 0.8 M KOH. Now, add sufficient volume of aqueous ammonia in order to dissolve the brown precipitate.
- Test sample
Materials required
- Test tubes
- Test tube stand
- Pipette
Equipment
- Water bath
Procedure of Tollens’ test
- Take two clean, dry test tubes and add 1 ml of the test sample in one test tube and 1 ml of distilled water in another as blank.
- Add 2 ml of Tollen’s reagent to both the test tubes.
- Keep both the test tubes in a water bath for 1 min.
- Observe the formation of color and note it down.
Result and Interpretation of Tollens’ test
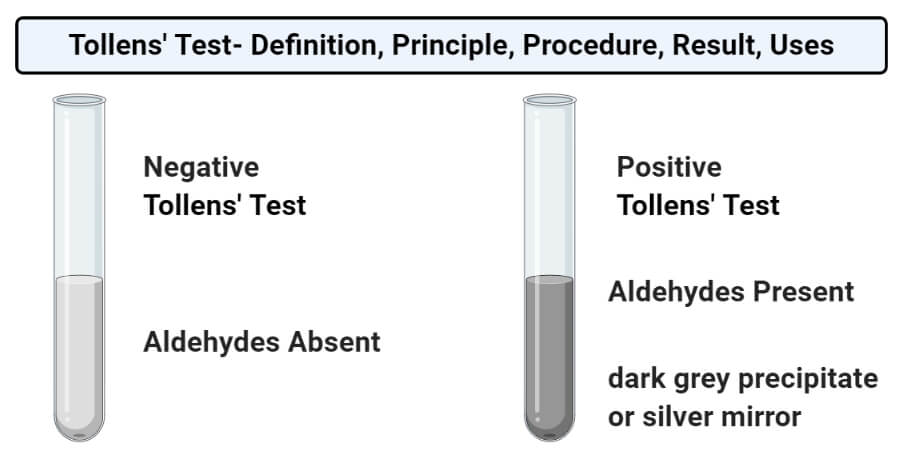
- The formation of a dark grey precipitate or silver mirror on the bottom and sides of the test tube indicates a positive result, which means that the given sample contains reducing sugars/ aldoses.
- The absence of such precipitate indicates a negative result, which means that the test sample doesn’t have reducing sugars/ aldoses/ α-hydroxy ketoses.
Uses of Tollens’ test
- Tollen’s test is routinely performed in chemical laboratories for the qualitative organic analysis, which distinguishes aldehydes from ketones.
- This test is also used for the differentiation of reducing sugars from non-reducing sugars.
Limitations of Tollens’ test
- Some carbohydrates that do not have an aldehyde group might give a positive result on Tollen’s test because of the isomerization of such sugars under alkaline conditions.
References and Sources
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- 3% – https://projects.ncsu.edu/project/chemistrydemos/Organic/TollensTest.pdf
- 2% – https://byjus.com/chemistry/tollens-test/
- 2% – https://byjus.com/chemistry/tests-of-carbohydrates/
- 1% – https://science.blurtit.com/658440/an-aqueous-solution-of-silver-nitrate-is-added-to-an-aqueous-solution-of-ironiichloride
- 1% – https://memberfiles.freewebs.com/31/91/47149131/documents/bio%20chem%20prelims%20hand%20outs.docx
- 1% – https://chemdemos.uoregon.edu/demos/Fehling-Test
- 1% – http://www.chem.boun.edu.tr/wp-content/uploads/2014/04/Chem-415-Experiment-1.pdf
