Starch, a polysaccharide, is a biodegradable natural carbohydrate that acts as an energy store in plants and serves the plant as a reserve food supply.
It is a staple carbohydrate in the human diet and plays a crucial role in quality and nutritional value improvement in the food industry.
Starch consists of glucose molecules synthesized by the green leaves of plants during photosynthesis and found in the form of granules in plants. When photosynthetic activity is inadequate, It breaks down to glucose and helps in nourishing the plant.
Interesting Science Videos
Sources of Starch
Starch is present in the leaves of green plants, stems (sago), roots of the cassava plant, all vegetables, fruits (banana, plantain), tubers (potatoes, cassava), cereals (such as wheat, corn, maize, sorghum and, rice), and some algae.
Structure of Starch
Starch or amylum is a homopolymer (each yields only one type of monosaccharide (glucose) after complete hydrolysis) composed of D-glucose units linked by α-(1→4) glycosidic bonds. The α-(1→4) glycosidic linkage between the glucose units is formed by starch synthases. It is also called glucosan or glucan. α, β -amylases specifically act on catalyzing the degradation of α-1,4 linkages. Starch hydrolyzes to liberate dextrins and then maltose and glucose units with the help of an amylase enzyme.
It constitutes two polysaccharide components: water-soluble amylose (20-30%) and water-insoluble amylopectin (70-80%).
Both components are present in the starch granules.
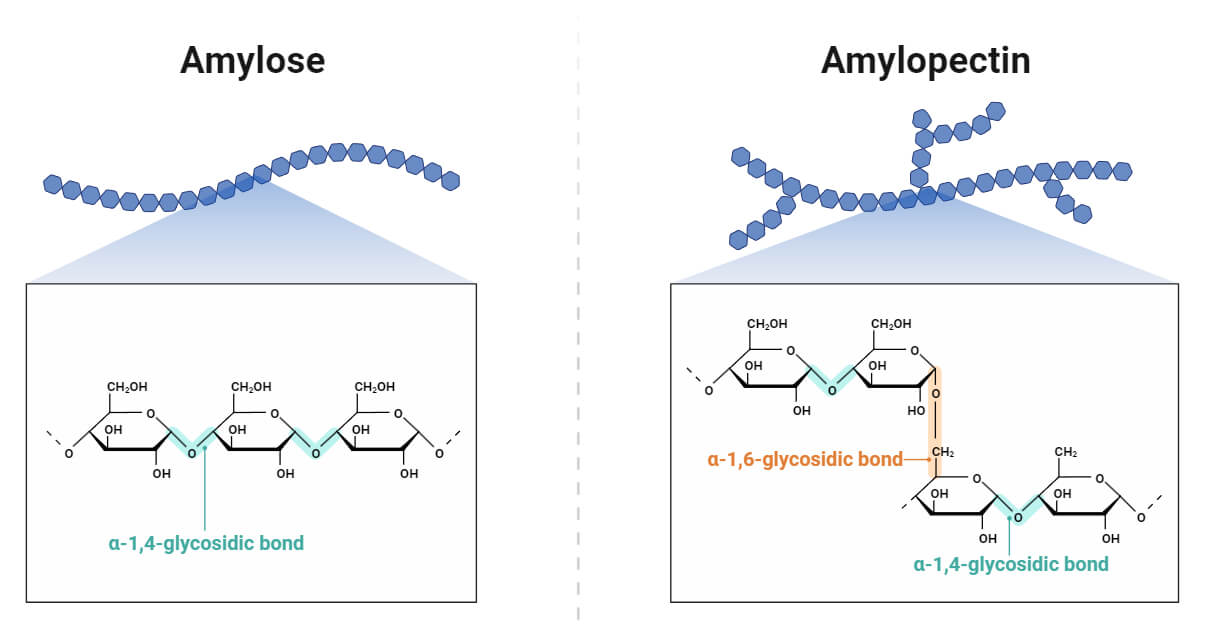
Amylose
Amylose, a linear molecule, is a long unbranched chain with 200- 1,000 α-D-glucopyranosyl units linked by α (1-4) glycosidic linkages. Amylose is amorphous in nature. Amylose leaches out of the granules, allowing water to enter and swell upon heating. Amylose compound produces a blue color when starch reacts with iodine. It is less soluble in water and does not form gel when hot water is added. The common source of amylose is a hybrid corn containing 50 % up to 70 % amylose. Other sources are rice and potato starches (up to 30%), quinoa, beans, bananas, and lentils.
Amylopectin
Amylopectin is a highly-branched polymer with 50,000 to 5,00,000 α-D-glucopyranosyl units linked by α (1-4) linkages everywhere and by α (1-6) glycosidic bonds at the branching points. It has a molecular weight of 107 to 108 daltons. Each branch constitutes 20-30 glucose units, and each molecule possesses hundreds of these branches. Amylopectin is crystalline in nature. The branching points in amylopectin join together to facilitate the formation of allomorphs. The bond formed in amylopectin is weaker than the linear amylose molecules. It is more soluble in water and forms a gel when hot water is added.
It influences viscosity changes due to water and heating.
A maximum of three hydroxyls are present in each glucose unit and are available for chemical substitution. It gives a purple color when reacting with iodine. The higher amount of amylopectin is present in grain rice, waxy potato starch, waxy corn, and glutinous rice, whereas lower in long-chain rice, amylomaize, and russet potatoes.
Properties of Starch
The properties depend on the molecular and structural composition of amylose and amylopectin, percent composition, and arrangement of these two homopolysaccharides in starch granules.
- It has good iodine-binding ability.
- It has good swelling power.
- It lowers water absorption capacity.
- It has high viscosity.
- It acts as Emulsifiers.
- Encapsulants
- Gelling ability or clouding agent
- Gelatinization
- Freeze-thaw and cold storage stabilities
- Binders
Uses of Starch
- Confectioneries, cough syrups- Act as anti-crystallizing
- Beverages- Act as a sweetening power
- Dairy Products- Act as a Bulking agent and improve texture
- Bakery Products- Act as moistening
- Soups and sauces- Act as a thickening and binding agent
- Paper- Increase strength
- Corrugated board- Acts as an adhesive agent
- Use for ethanol, polyurethane, and bioplastics production
- Act as a binding capacity in glues and adhesives
- Cosmetics- Act as Emulsifier
- Jams- Act as a preservative agent
- Freezing point depression in ice creams
- Provide nutritional quality in baby food and clinical nutrition
- Animal Feeds- Palatability for pet food, high digestibility for piglets starter feed, milk powder replacement in calf milk, and yellow pigmentation for egg yolks
Types of Starch
There are two types of starch. They are:
- Native Starch
- Processed/ Modified Starch
A. Native Starch
Native Starches are long-chain carbohydrates which are pure forms of starch. It exists in plant sources such as corn, maize, rice, wheat, potato, cassava, and tapioca. The extraction of pure starches from sources produces white, tasteless, odorless, either powder or liquid. They are insoluble in cold water and swell to different degrees depending on type and temperature.
In this group, starch has not altered to any other treatments, like physically, chemically, or enzymatically. Despite being used in the food industry for decades, its industrial application is limited because of its inherent properties, such as resistance to high temperature or acid (starch breaks down when reheated or in an acidic environment) and poor thermal stability.
Native starches have tremendous properties such as thickening, texturizing, gelling, moisture retention, antistaling properties, stabilizing, film forming, dusting, and dough binding.
Applications of Native Starch
- Bakery mixes
- Frozen cakes
- Sheeted snacks
- Batters & breadings
- Brewing adjuncts
- Licorice confections
- Dry mix soups and sauces
- Pet foods
- Processed meat
- Pudding powders
- Cold Process Salad dressings and dips
- Frozen prepared entrée sauces
- Fruit Preps
B. Processed/Modified Starch
The physiochemical and functional properties of native starch have been altered or modified with any other treatments like physical, chemical, enzymatic, or combination of these as per the Good Manufacturing practices for improved physiochemical characteristics, functionalities, and nutritional qualities and to change, strengthen, or impair new properties by molecular cleavage, re-arrangement is known as modified starch. Due to this, there will be changes in the properties such as gelatinization temperature, viscosity, retrogradation (a reaction occurs in gelatinized starch when the amylose and amylopectin chains realign themselves), gel clarity, texture, and taste of native starch.
Modified starch fulfills the flaws of native starch. In food industries, unprocessed or original native starches are less preferred due to low thermal and shear resistance, poor resistance to acid and alkali, resistance to freeze/thaw, instability shear stress, viscosity loss while processing, and higher retrogradation tendency. Because of this limitation, the quality of food products deteriorates. That is why modification of starch is needed to improve nutritional quality and to increase shelf life.
Sources of modified Starch
The most common sources of native starch used in this modification are various roots, tubers, cereals, and legumes. The most common types of modified food starch obtained from corn, wheat, potato, and tapioca are as follows:
- Modified corn starch
- Modified waxy maize starch
- Modified tapioca starch
- Modified potato starch
- Modified wheat starch
- Modified rice starch
Modified starches have properties like thickening, stabilizing, emulsifying, texturizing, taste, gel clarity, resistance to acid and alkali, shear, resistance at different temperatures (excessive heat, freezing), retrogradation (expel water from the polymer network), and increased shelf life.
Types of modification methods
There are three types of modification methods:
- Physical modification
- Chemical modification
- Enzymatic modification
- Genetic modification
1. Physically modification
Based on physical factors (moisture, temperature, pressure, pH change, radiation treatment, and ultrasonic treatment), it changes the functional, morphological, physical, and structural characteristics of starch, such as solubility, crystallinity, swelling power, viscosity, thermal snd stability properties. It helps starch to swell and to be soluble in cold water.
The commonly used methods are superheating of starch, thermal inhibition treatment, UV and gamma irradiation, microwave treatment, high pressure, osmotic pressure, instantaneous controlled pressure treatment, mechanical activation by stirring ball mill, pulsed electric field treatment, micronization in vacuum ball mill, annealing, and freeze-thaw treatment.
Applications of Physically modification
- It helps in the production of modified starch and hydrolyzed starch.
- It acts as a thickening and binding agent.
- It helps in controlling moisture.
- Salad dressings
- Toppings
- Syrups
- Yogurt
2. Chemical Modification
It also modifies the physiochemical characteristics of starch by adding new chemical or functional groups in starch despite not altering the shape and size of starch granules (starch deposited in plants in the form of granules). Each anhydroglucose unit (AGU) consists of three hydroxyl groups adjacent to the carbon atoms at positions 2, 3, and 6. This arrangement renders the ability to alter starch easily through a chemical reaction with different functional groups.
The chemical modification includes esterification and etherification, emulsification, cationization, oxidation, acid treatment, and cross-linking (making a starch more heat resistant). Conventional chemical methods use acids and acetates, hypochlorites, and phosphates. Mostly, stabilized and cross-linked starch is implemented in food. These modifications greatly expand the field of potential applications in the food industry. However, this treatment can be pernicious to the environment and requires recycling. So, the enzymatic method is preferred more.
Functions of Chemical Modification
- Improve the gel setting performance and lower gelatinization temperature
- Increase the stability during frozen storage
- Reduce retrogradation
Applications of Chemical Modification
- Additives for medicine, cosmetics, coatings, oil exploiting
- Drug delivery
- Packaging biofilm (green packaging)
- edible films
- Food additives (like stabilizers and thickeners such as hydroxypropyl starch and acid-treated starch)
- Papermaking
- Fat replacers in ice cream, salad dressings, cheese, baked foods, icings, mayonnaise
- eliminate oil uptake by the foods
- Improve dough characteristics
- Canned foods, bakery and frozen foods, sauces, ketchup, jam, and jellies- act as pectin replacers in these food products
3. Enzymatic Modification
Enzyme treatment is a direct modification of starch structure, which means there will be changes in molecular size and weight, the ratio of amylose to amylopectin, and branch chain length distribution. When activation energy gets lower and the reaction is mild, the complex chains of starch converts into simple sugars like maltose with the help of enzymes. This process is also called enzymolysis. The enzyme can be retrieved and reused. This method replaced the physical and chemical methods in producing the modified porous starch. Moreover,
It is innocuous to the environment as well as food consumers. For example, Starch retrogradation referred to as staling, which is a core problem of starchy-based foodstuffs such as bread and rice cake. To limit the retrogradation rate, improving instability of the gelatinized starch structure, viscosity loss, various enzymes, emulsifiers, oligosaccharides, and polysaccharides have been initiated.
Functions of Enzymatic Modification
- Lowers energy cost
- Offers higher yield
- specific reactions for starch substrate
Important starch-modifying enzymes
Starch-modifying enzymes are classified into two families. They are Glycoside hydrolase and transglycosylase for starch functionalization.
- Glycoside hydrolase: It is also known as glycosyl hydrolases or glycosidases. These enzymes catalyze the hydrolysis of glycosidic linkage of glycosides, which leads to the formation of a sugar hemiacetal (glucose) or hemiketal and the free aglycon. The glycosyl enzyme intermediate can react with water to cause hydrolysis is called glycoside hydrolase activity. It includes enzymes such as α-amylase (hydrolases that hydrolyze α-1. 4-glycosidic linkage of starch to produce oligosaccharides), β-amylase (hydrolyzes α-1,4-glycosidic linkages in starch and glycogen with an inversion of configuration on the C (1) position of the glucose from α to β forming maltose.), branching enzyme (glucan transferase), de-branching enzymes (pullulanase, isoamylase), cyclodextrin glycosyltransferase, amylomaltase and 4-α-glucanotransferase.
- Transglycosylase: These enzymes catalyze the transformation of one glycoside to another. The activity in which glycosyl enzyme intermediate reacts with a sugar acceptor to cause transglycosylation is called transglycosylase activity.
These starch-modifying enzymes are isolated from micro-organisms including Thermus thermophilus, Thermus scotoductos, Thermus aquatieus, Bacillus stearothermophils, Bacillus acidopullulyticus, Aspergillus niger, Bacillus subtilis, Bacillus magaterium, Alkalophilc Bacillus, Neisseria polysaccharides, Deinococcus geothermalis, Rhodothermus obamensis, Aquifex aeolicus, Deinococcus radiodurans
Some enzymes (usually β-amylase) are also isolated from various plant sources such as Ramie leaf and Barley (malt).
Applications of Enzymatic Modification
- Production of organic acids (citric, lactic, and malic acid)
- Production of sweeteners such as maltodextrin, corn syrup, dextrose syrup, oligosaccharides, crystalline dextrose, and fructose
- Production of Polyols (mannitol, sorbitol, maltitol, and xylitol); Amino acids (cysteine, threonine, lysine, tryptophan, and methionine); alcohol (ethanol)
- Baking industry such as bread or rice cake for improving quality (crumb softness in bread, increases shelf life, production efficiency)
4. Genetic Modification
Genetic modification employs amylose-free starch, high amylose starch, and starch with altered amylopectin structure.
In a genetic modification of starch, transgenic technology is implemented to specifically target the enzymes involved in the pathways leading to the biosynthesis and degradation of starch in plants.
Applications of Starch Modification
- Canned food
- Frozen prepared foods
- Meat products: sausage, canned meat
- Bakery: bread, cake, biscuits
- Confectionary: soft candy, gelling candy, jelly- forming a hard shell
- Beverage
- Dairy desserts: yogurt, ice cream, pudding
- Fruit pie and cream fillings
- dressings- act as an emulsifier and stabilizer
- Sauce (flavored sauce, cheese sauce) and Gravies- decrease lumpy consistency when mixed
- Instant food: noodles
- Soups- act as a thickener
References
- https://www.medicalnewstoday.com/articles/what-is-starch#what-it-is
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6400748/
- https://link.springer.com/referenceworkentry/10.1007/978-3-540-30429-6_35
- https://www.slideshare.net/GeetaraniLoushigam/starch-presentation-244338258
- https://www.cargill.com/doc/1432087949297/back-to-basics-native-starches.PDF
- https://www.bobsredmill.com/blog/healthy-living/modified-food-starch-demystified/
- https://foodadditives.net/starch/modified-food-starch/
- https://link.springer.com/article/10.1007/s11947-022-02761-z
- https://www.researchgate.net/publication/340594422_ENZYMATIC_MODIFICATION_OF_STARCH
- https://www.slideshare.net/gideonattwell/what-is-starch-14185449
- https://www.researchgate.net/publication/339887938_Physical_and_Chemical_Modifications_in_Starch_Structure_and_Reactivity
- https://www.slideshare.net/SyedAdilHasanRizvi/starch-250635868
- https://vivadifferences.com/difference-between-amylose-and-amylopectin/
- https://www.livestrong.com/article/465067-high-amylose-foods/

Hi Dear Prativa Shrestha
I’m Ghasemi, and I;m looking researches and knowledge about corn(maize) starch in poultry feeds. could you help me?
Emai; a.gassemi_64@yahoo.com