The sodium-potassium (Na+/K+) pump or Na+/K+ adenosine triphosphatase (ATPase) is an electrogenic transmembrane enzyme that maintains the resting membrane potential across the cell membrane.
It is mostly observed in nerve cells for neurotransmission through electrical signals. It was first discovered in 1957 by the Danish scientist Jens Christian Skou, who was later awarded the Nobel Prize in 1997. This discovery improved our understanding of cell ion intake and outflow.
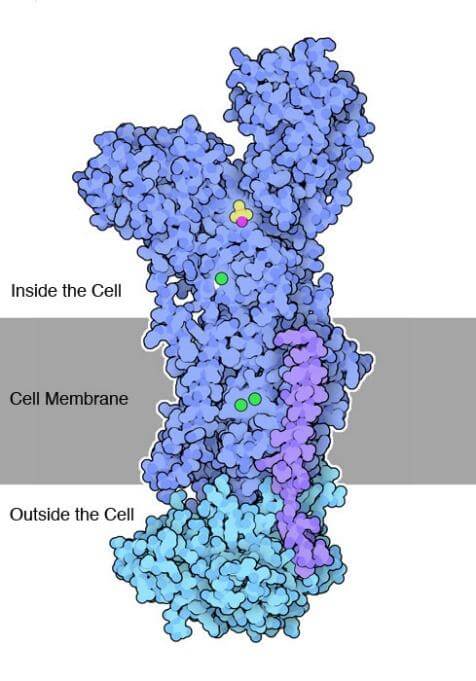
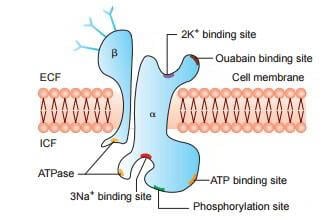
Structure of Enzyme
The Na+/K+ ATPase pump comprises a catalytic alpha subunit and an auxiliary beta subunit. The alpha subunit consists of a transmembrane region composed of about 10 helices known as MA1-M10. The helices hold three Na+ ion binding sites in the E1 state and two K+ binding sites in the E2 state. Similarly, the entire structure of the Na+/K+ ATPase is divided into three sites. The 1st site and the 2nd site overlap within the E1 and E2 states. The E3 site is solely in the E1 state between helices M5 and M6. Depending on the concentrations of the ions, this site can also bind to Na+ and catalyze H+ for transport.
Functions of Sodium-Potassium (Na+/K+) Pump
In living cells, there is a higher concentration of K+ ions intracellularly and a lower concentration of Na+ ions extracellularly. This is because the enzymatic pump transports three sodium (Na+) ions out of the cell and two potassium (K+) ions into the cell for every ATP molecule hydrolyzed. The Na+/K+ ATPase pump functions against the concentration gradient (active transport). For this reason, it requires energy in the form of ATP hydrolysis. This helps generate and maintain an osmotic equilibrium and membrane potential in the cells.
Most cells utilize almost 1/3rd of their generated ATP in the functioning of this enzyme. In neurons, the energy consumption is even larger because of neurotransmission. Similarly, the Na+/K+ ATPase pump is significantly important in glycolysis in skeletal muscles, where inhibition of glycogenolysis leads to reduced Na+/K+ pump activity.
Resting potential
The movement of three sodium (Na+) ions extracellularly and two potassium (K+) ions intracellularly develops the resting membrane potential. This leads to the depletion of sodium ions and an increase of potassium ions in the cell. The permeability of passive K+ transport in the extracellular matrix is more rapid than the passive transfer of Na+ inside the cell. Therefore, there is a net movement of positive charge to the outside of the cell, generating the resting membrane potential. At steady state, the resting membrane potential may vary between -60 mV to -70 mV, depending on the cell type.
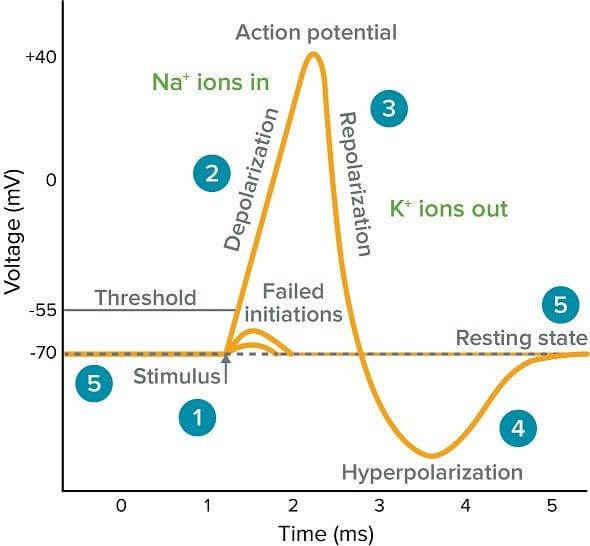
Active potential
When a stimulus or a disturbance upsets the resting membrane potential, causing a transient change in the membrane potential, then this is called an action potential. The action potential is the process by which neurons send signals to other neurons or muscle cells. The process is non-simultaneous and is carried out in a localized spot where the disturbance has occurred. The potential generally exceeds about +40 mV.
In active potential, the permeability of Na+ channels (low in the resting state) increases rapidly, and the Na+ ions flood into the cell. The membrane potential is more positive in the cytosol, a process known as depolarization. After a short duration, the K+ channels are also opened, letting the K+ ions out of the cell, restoring the original resting potential, a process known as repolarization. After that, there is an increased permeability to K+ ions, allowing them to move outside of the cell, therefore, making the cell even more negatively charged than the resting potential state. This results in hyperpolarization. Hyperpolarization is important for the prevention of cellular re-firing, i.e., maintaining a refractory period before the intervention of another active potential. It also ensures one-way transmission of nerve impulses and sets a limit on the frequency of neuron transmission.
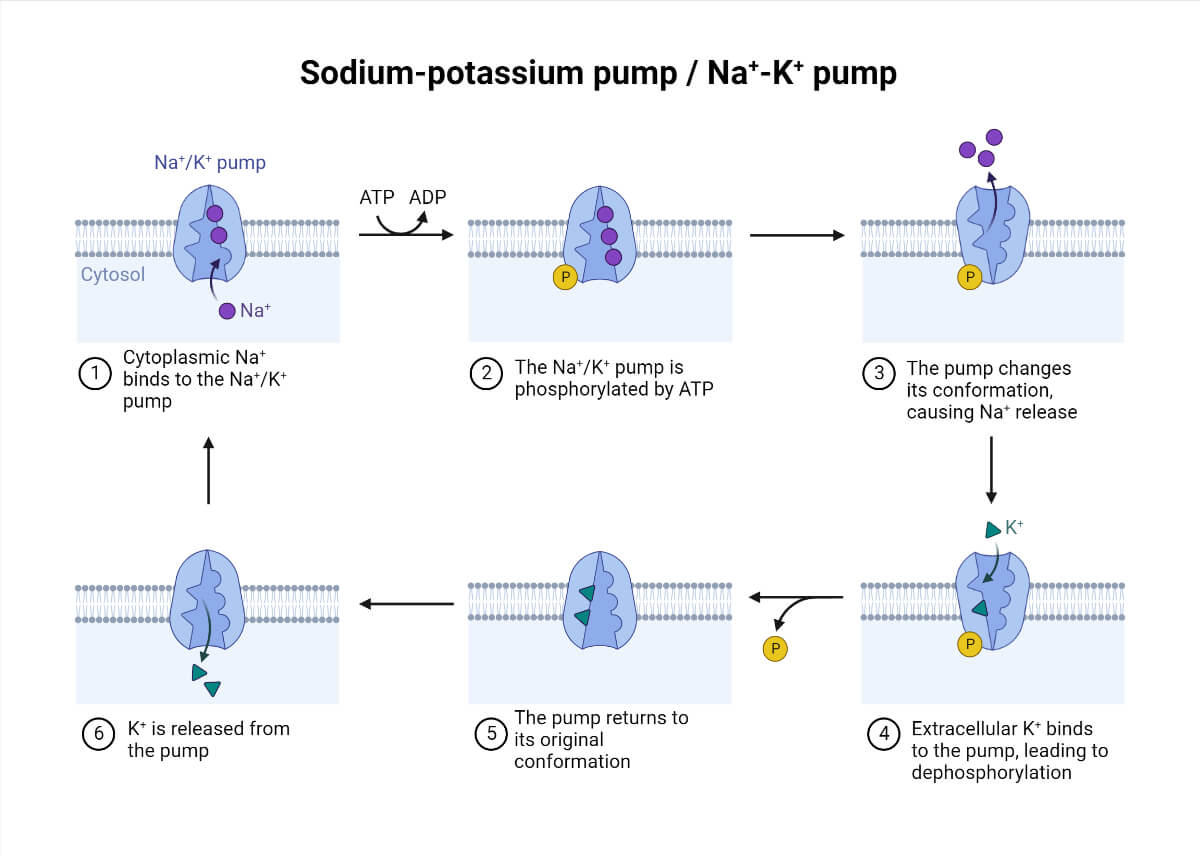
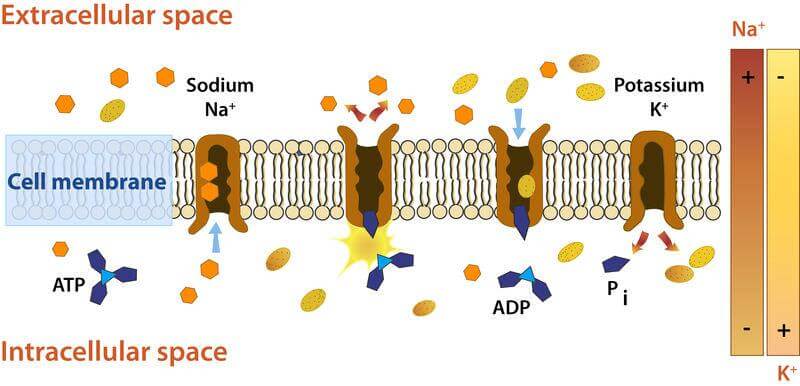
Mechanism of Sodium-Potassium (Na+/K+) Pump
The enzymatic reaction of the Na+/ K+ ATPase pump is as follows:
- The pump has a higher affinity for Na+ ions than K+ ions after binding to ATP. This leads to the binding of three intracellular Na+ ions.
- Once the ATP is hydrolyzed to ADP, there is a conformational change in the pump because of phosphorylation.
- The conformational change exposes the Na+ ions to the extracellular region. This phosphorylated form has a lower affinity for Na+ ions; therefore, they are released.
- The pump binds to two K+ ions, reverting to the previous state and releasing the ions into the cell.
- The process is repeated, maintaining the resting potential.
Significance of Sodium-Potassium (Na+/K+) Pump
- The Na+/K+ ATPase pump is essential for stabilizing the cell’s resting membrane potential and also for the production of neuronal action potential.
- Sperm cells utilize the Na+/K+ ATPase to regulate membrane potential for sperm motility and the acrosome functioning for penetration of the egg.
- Nephrons of the distal convoluted tubule in the kidneys comprise a large number of the ATPase enzyme (50 million per cell) to filter waste products in the blood, reabsorb glucose, reabsorb amino acids, maintain pH, and regulate electrolyte levels in the blood.
- The failure of the Na+/K+ pump can result in swelling of the cell because of osmosis (higher ion concentration results in water inside the cell). This can eventually cause the cell to lyse.
References
- 2.16: Sodium-Potassium Pump. (2016, September 20). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Introductory_Biology_(CK-12)/02%3A_Cell_Biology/2.16%3A_Sodium-Potassium_Pump
- Action potential. (n.d.). Kenhub. Retrieved April 10, 2025, from https://www.kenhub.com/en/library/physiology/action-potential
- Active Transport – Primary and Secondary Processes. (2018, August 8). Earth’s Lab. https://www.earthslab.com/physiology/active-transport/
- Betts, J. G., Young, K. A., Wise, J. A., Johnson, E., Poe, B., Kruse, D. H., Korol, O., Johnson, J. E., Womble, M., DeSaix, P., Betts, J. G., Young, K. A., Wise, J. A., Johnson, E., Poe, B., Kruse, D. H., Korol, O., Johnson, J. E., Womble, M., & DeSaix, P. (2013, April 25). 3.1 The Cell Membrane—Anatomy and Physiology | OpenStax. OpenStax. https://openstax.org/books/anatomy-and-physiology-2e/pages/3-1-the-cell-membrane
- Depolarization, hyperpolarization & neuron action potentials (article) | Khan Academy. (n.d.). Retrieved April 10, 2025, from https://www.khanacademy.org/science/biology/human-biology/neuron-nervous-system/a/depolarization-hyperpolarization-and-action-potentials
- PDB101: Molecule of the Month: Sodium-Potassium Pump. (n.d.). RCSB: PDB-101. Retrieved April 10, 2025, from http://pdb101.rcsb.org/motm/118
- What is Action Potential, Membrane Potential, Action Potential Chart. (n.d.). Retrieved April 10, 2025, from https://www.moleculardevices.com/applications/patch-clamp-electrophysiology/what-action-potential
