- A randomized controlled trial (or randomized control trial; RCT) is a type of scientific (often medical) experiment that aims to reduce certain sources of bias when testing the effectiveness of new treatments.
- It is a trial in which subjects are randomly assigned to one of two groups: one (the experimental group) receiving the intervention that is being tested, and the other (the comparison group or control) receiving an alternative (conventional) treatment.
- The two groups are then followed up to see if there are any differences between them in the outcome.
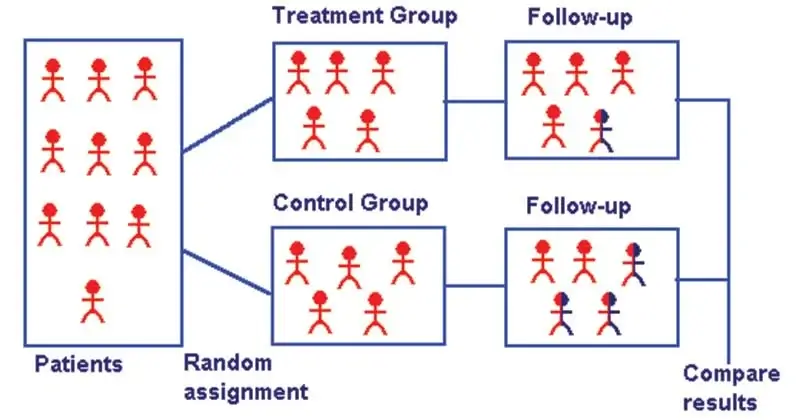
Image Source: http://www.edinburgh-eyetests.co.uk/ebm.htm
Interesting Science Videos
Features of Randomized Controlled Trials (RCTs)
- They are randomized: The researchers decide randomly as to which participants in the trial receive the new treatment and which receive a placebo, or fake treatment.
- They are controlled: The trial uses a control group for comparison or reference. In the control group, the participants do not receive the new treatment but instead receive a placebo or reference treatment.
Principle of Randomized Controlled Trials (RCTs)
- It is accomplished by randomly allocating subjects to two or more groups, treating them differently, and then comparing them with respect to a measured response.
- One group—the experimental group—has the intervention being assessed, while the other—usually called the control group—has an alternative condition, such as a placebo or no intervention.
- The groups are followed under conditions of the trial design to see how effective the experimental intervention was.
- Treatment efficacy is assessed in comparison to the control.
- There may be more than one treatment group or more than one control group.
- The results and subsequent analysis of the trial are used to assess the effectiveness of the intervention, which is the extent to which treatment, procedure, or service does patients more good than harm.
Steps in Randomized Controlled Trials (RCTs)
The basic steps in conducting an RCT include the following:
- Drawing up a protocol.
- Selecting reference and experimental populations.
- Randomization.
- Manipulation or intervention.
- Follow-up.
- Assessment of outcome
1. The protocol
- One of the essential features of a randomized controlled trial is that the study is conducted under a strict protocol.
- The protocol specifies the aims and objectives of the study, questions to be answered, criteria for the selection of study and control groups, size of the sample, the procedures for allocation of subjects into study and control groups, treatments to be applied when and where and how to what kind of patients, standardization of working procedures and schedules as well as responsibilities of the parties involved in the trial, up to the stage of evaluation of outcome of the study.
- The protocol aims at preventing bias and to reduce the sources of error in the study.
2. Selecting reference and experimental populations
(a) Reference or target population:
- It is the population to which the findings of the trial, if found successful, are expected to be applicable (e.g., a drug, vaccine or other procedure).
- A reference population may be as broad as mankind or it may be geographically limited or limited to persons in specific age, sex, occupational or social groups.
(b) Experimental or study population:
- The study population is derived from the reference population. It is the actual population that participates in the experimental study.
- Ideally, it should be randomly chosen from the reference population, so that it has the same characteristics as the reference population.
- If the study population differs from the reference population, it may not be possible to generalize the findings of the study to the reference population.
The participants or volunteers must fulfill the following three criteria:
- They must give “informed consent”, that is they must agree to participate in the trial after having been fully informed about the purpose, procedures and possible dangers of the trial;
- They should be representative of the population to which they belong (i.e., reference population); and
- They should be qualified or eligible for the trial.
3. Randomization
- Randomization is a statistical procedure by which the participants are allocated into groups usually called “study” and “control” groups, to receive or not to receive an experimental preventive or therapeutic procedure, maneuver or intervention.
- Randomization is an attempt to eliminate “bias” and allow for comparability.
- Randomization is the “heart” of a controlled trial. It will give the greatest confidence that the groups are comparable so that “like can be compared with like”.
- Randomization is done only after the participant has entered the study that is after having been qualified for the trial and has given his informed consent to participate in the study.
- Randomization is best done by using a table of random numbers.
4. Manipulation
- Having formed the study and control groups, the next step is to intervene or manipulate the study (experimental) group by the deliberate application or withdrawal or reduction of the suspected causal factor (e.g., this may be a drug, vaccine, dietary component, a habit, etc) as laid down in the protocol.
- This manipulation creates an independent variable (e.g., drug, vaccine, a new procedure) whose effect is then · determined by measurement of the final outcome, which constitutes the dependent variable (e.g., the incidence of disease, survival time, recovery period).
5. Follow-up
- This implies examination of the experimental and control group subjects at defined intervals of time, in a standard manner, with equal intensity, under the same given circumstances, in the same time frame till final assessment of outcome.
- The duration of the trial is usually based on the expectation that a significant difference (e.g., mortality) will be demonstrable at a given point in time after the start of the trial.
- Thus the follow-up may be short or may require many years depending upon the study undertaken.
6. Assessment
The final step is the assessment of the outcome of the trial in terms of:
(a) Positive results: that is, benefits of the experimental measures such as reduced incidence or severity of the disease, cost to the health service, or other appropriate outcomes in the study and control groups.
(b) Negative results: that is, severity and frequency of side-effects and complications, if any, including death. Adverse effects may be missed if they are not sought.
- The incidence of positive/negative results is rigorously compared in both the groups and the differences, if any, are tested for statistical significance.
- Techniques are available for the analysis of data as they are collected (sequential analysis), but it is more useful to analyze the results at the end of the trial.
Potential Bias in RCTs
Bias may arise from errors of assessment of the outcome due to the human element. These may be from three sources:
- First, there may be bias on the part of the participants, who may subjectively feel better or report improvement if they knew they were receiving a new form of treatment. This is known as “subject variation”.
- Secondly, there may be observer bias that is the investigator measuring the outcome of a therapeutic trial may be influenced if he knows beforehand the particular procedure or therapy to which the patient has been subjected. This is known as “observer bias.”
- Thirdly, there may be bias in evaluation that is, the investigator may subconsciously give a favorable report of the outcome of the trial.
The Blinding Technique
In order to reduce these problems, a technique known as “blinding” is adopted, which will ensure that the outcome is assessed objectively.
Blinding can be done in three ways:
(a) SINGLE BLIND TRIAL: The trial is so planned that the participant is not aware whether he belongs to the study group or control group.
(B) DOUBLE-BLIND TRIAL: The trial is so planned that neither the doctor nor the participant is aware of the group allocation and the treatment received.
(C) TRIPLE BLIND TRIAL: This goes one step further. The participant, the investigator, and the person analyzing the data are all “blind”.
Ideally, of course, triple blinding should be used; but double-blinding is the most frequently used method when a blind trial is conducted.
Significance of Randomized Controlled Trials (RCTs)
- Randomized controlled trials are the most reliable method available for testing new treatments.
- RCTs are the most stringent way of determining whether a cause-effect relation exists between the intervention and the outcome
- They have become the standard that pharmaceutical companies must meet for calculating and proving the level of efficacy and safety of an experimental drug.
References
- https://emj.bmj.com/content/20/2/164
- https://www.medicalnewstoday.com/articles/280574.php
- Park, K. (n.d.). Park’s textbook of preventive and social medicine.
- Beaglehole, Robert, Bonita, Ruth, Kjellström, Tord & World Health Organization. (1993). Basic epidemiology, Updated reprint. World Health Organization.
- Gordis, L. (2014). Epidemiology (Fifth edition.). Philadelphia, PA: Elsevier Saunders.
- Hennekens CH, Buring JE. Epidemiology in Medicine, Lippincott Williams & Wilkins, 1987.
- White, F., Stallones, L., & Last, J. M. (2013). Global public health: Ecological foundations. New York, NY: Oxford University Press.
