Interesting Science Videos
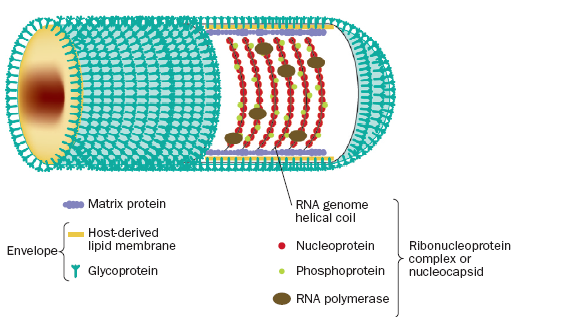
Structure of Rabies Virus
- The virus is enveloped, rod shaped particles measuring 75 × 180 nm.
- Mature virion appears either as bullet shaped particles with one rounded and one flattened end or as bacilliform particles.
- The particles are surrounded by a membranous lipid envelope.
- The particles have a buoyant density in CsCl of about 1.19 g/cm3.
- The virion outer surface is covered with protruding spikes which is 10nm long.
- The peplomers (spikes) are composed of trimers of the viral glycoprotein.
- Inside the envelope is a ribonucleocapsid which encloses single-stranded, negative-sense RNA genome (12 kb; molecular weight 4.6 *10ˆ6).
- The genome encode for five proteins designated as glycoprotein(G), nucleoprotein(N), phosphoprotein(P), matrix protein(M) and large polymerase protein(L).
Genome of Rabies Virus
- The rabies virus genome is composed of approximately 12000 nucleotides.
- It is single-stranded RNA, linear, non-segmented, negative sense.
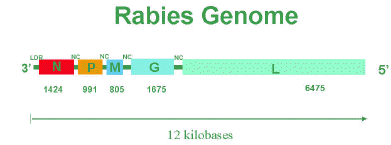
- The genomic RNA encodes for the five different viral proteins: nucleoprotein(N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and the large polymerase protein (L).
- The viral ribonucleoprotein (RNP) core consists of the viral RNA encapsidated by N proteins and associated with the P and L proteins.
- The other viral proteins, M and G are involved in virion structure and attachment to cellular receptors, respectively.
- The intergenic region (G-L) is approximately 450 nucleotides in length and does not appear to encode any polypeptides.
Epidemiology of Rabies Virus
- Rabies infection in man is generally acquired from the bite of an infected animal.
- The domestic dog (Canis familiaris) is the most important vector, although bat rabies continues to cause human infection across much of Central and South America.

- The virus still remains endemic across much of the developing world, where the majority (99%) of human deaths due to rabies occur, mainly in Africa and Asia although extensive vaccination campaigns in dog and terrestrial wildlife populations have reduced the incidence across globe.
- The World Health Organization (WHO) estimates an annual toll of 55 000 deaths following human infection with rabies virus, although this is likely to be a gross underestimate.
Transmission of Rabies Virus
- Bite of infected rabid animal
- Contact of saliva with broken skin or with mucous membrane
- Corneal transplant
Replication of Rabies Virus
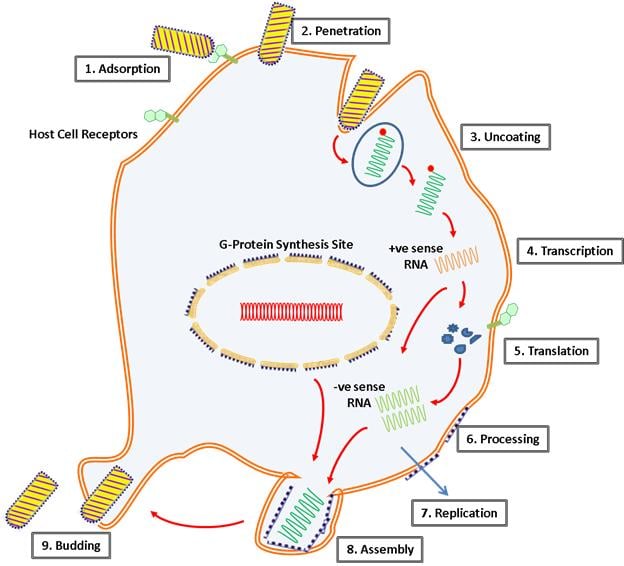
- Infection spreads through the bite of infected rabid animal.
- A significant interaction of G protein and acetylcholine receptors of peripheral nervous system provide evidence of virus attachment.
- The virus then gains entry to central nervous system, entry mediated through mechanism called endocytosis.
- Within the endosome, the pH lowers which bring about the conformational changes in the G protein that promotes the fusion of viral membrane to endosomal membrane resulting in release of viral nucleocapsid.
- The single-stranded RNA genome is transcribed into positive sense mRNA by the virion-associated RNA polymerase.
- The monocistronic mRNAs code for the five virion proteins: nucleocapsid (N), polymerase proteins (L, P), matrix (M), and glycoprotein (G).
- Although G protein synthesis is initiated on free ribosomes, completion of synthesis and glycosylation (processing of the glycoprotein), occurs in the endoplamsic reticulum (ER) and Golgi apparatus.
- The genome RNP is a template for complementary positive-sense RNA, which is responsible for the generation of negative-sense progeny RNA.
- During the assembly process, the N-P-L complex encapsulates negative-stranded genomic RNA to form the RNP core, and the M protein forms a capsule, or matrix, around the RNP.
- The RNP-M complex migrates to an area of the plasma membrane containing glycoprotein inserts, and the M-protein initiates coiling.
- The M-RNP complex binds with the glycoprotein, and the completed virus buds from the plasma membrane.
- The viral matrix protein forms a layer on the inner side of the envelope, whereas the viral glycoprotein is on the outer layer and forms the spikes.
Pathogenesis of Rabies Virus
- After inoculation of infectious saliva by bite, virus may persist and replicate in muscle tissue before progressing to the peripheral nervous tissue via neuromuscular junction.
- Neurotropsim is a main feature associated with viral replication residing exclusively to neurons.
- A significant interaction of G protein and acetylcholine receptor provide evidence of viral attachment.
- After peripheral nerve entry, virus moves centripetally within axons to the CNS via transportation by retrograde axonal flow.
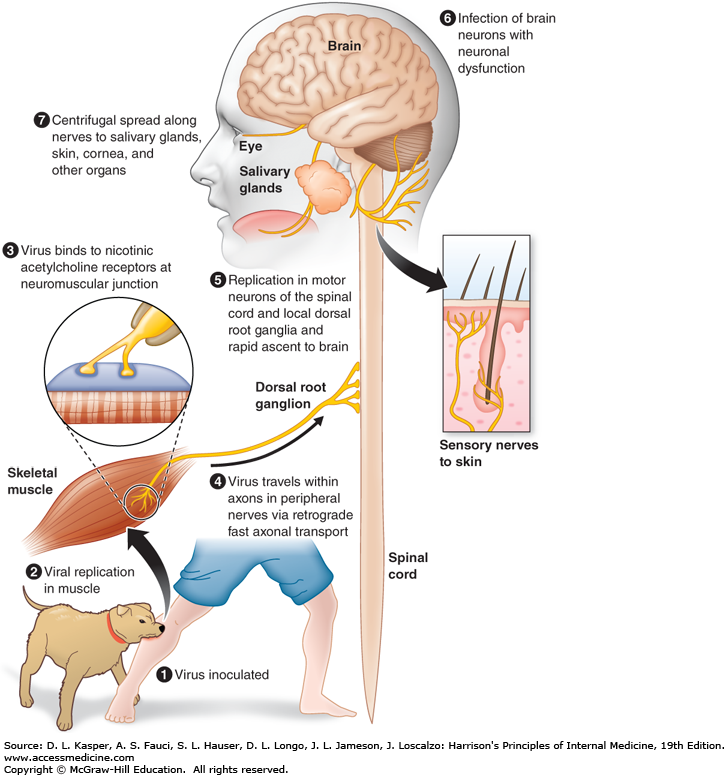
- Incubation period is dependent on the distance between site of bite and CNS.
- Apart from this, it also depends on age of host, immune status of host, viral strain involved and amount of inoculation.
- In the CNS, the multiplication of the virus occurs in the grey matter and spreads in the endoneurium of Schwann cell.
- Virus spread may be facilitated by movement across cell to cell junctions.
- After period of multiplication, it disseminate into tissues and organs via efferent neurons.
Clinical manifestations of Rabies Virus
- The disease is an acute, fulminant, fatal encephalitis.
- The incubation period in humans is typically 1–3 months but may be as short as 1 week or more than a year.
- The clinical spectrum can be divided into three phases: a short prodromal phase, an acute neurologic phase, and coma.
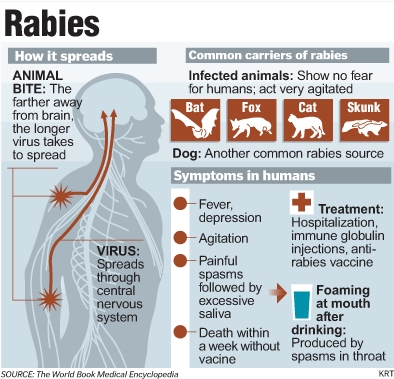
- The prodrome, lasting 2–10 days, may show any of the following nonspecific symptoms: malaise, anorexia, headache, photophobia, nausea and vomiting, sore throat, and fever.
- During the acute neurologic phase, which lasts 2–7 days, patients show signs of nervous system dysfunction such as nervousness, apprehension, hallucinations, and bizarre behavior.
- Beside these, general sympathetic overactivity is observed, including lacrimation, pupillary dilatation, and increased salivation and perspiration.
- Hydrophobia (fear of water) or aerophobia (fear when feeling a breeze) is exhibited by number of patients.
- The act of swallowing precipitates a painful spasm of the throat muscles.
- Neurologic phase is followed by convulsive seizures or coma and death.
- The major cause of death is cardiorespiratory arrest.
- The disease course is slower, with some patients surviving 30 days.
- However, recovery and survival are extremely rare.
Diagnosis of Rabies Virus
Specimen: saliva, corneal biopsy, brain tissue, neck skin biopsy
Histopathology
- Detection of Negri bodies by Seller’s staining technique which comprises use of basic fuchsin and methylene blue.
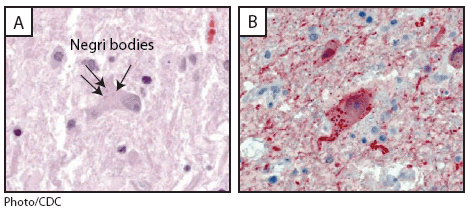
- Negri bodies are purplish pink, sharply demarcated, more or less spherical, and 2–10 μm in diameter, and they have a distinctive internal structure with basophilic granules in an eosinophilic matrix.
Antigen detection
- Tissues infected with rabies virus are currently identified most rapidly and accurately by means of immunofluorescence or immunoperoxidase staining using antirabies monoclonal antibodies.
Antibody detection
- Antibodies develop slowly in infected persons or animals during progression of the disease but promptly after vaccination with cell-derived vaccines.
- Serum antibodies to rabies can be detected by immunofluorescence or neutralization tests.
Virus isolation
- Available tissue is inoculated intracerebrally into suckling mice.
- Infection in mice results in encephalitis and death.
- The central nervous system of the inoculated animal is examined for Negri bodies and rabies antigen.
- However, virus isolation takes too long to be useful in making a decision about whether to give vaccine.
Molecular method
- Reverse transcription-polymerase chain reaction testing can be used to amplify parts of a rabies virus genome from fixed or unfixed brain tissue or saliva.
- Sequencing of amplified products using Nucleic Acid Sequence Based Amplification can allow identification of the infecting virus strain.
Treatment of Rabies Virus
- No successful treatment in clinical use.
Management, Prevention and Control of Rabies Virus
- This include preexposure and post exposure prophylaxis.
- Preexposure prophylaxis is given to individuals who are at risk that include veterians, animal handlers, laboratory workers.
- Human diploid cell line vaccine (HDCV) is given at two doses at 4 weeks interval.
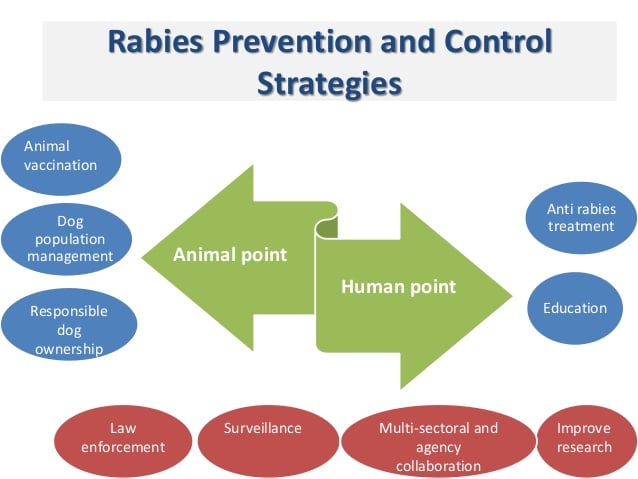
- Post exposure prophylaxis includes
- Animal examination for 10days for symptoms and are then killed.
- Wound management by surgical debrigement and cleaning of wound with soap and water and quaternary ammonium compounds.
- Passive immunization using Human Rabies Immune globulin (HRIG) collected from immunized persons and infiltrated at site of wound or administered intramuscularly as soon as possible after rabies exposure.
- Active immunization with modern tissue culture vaccines consists of a series of four doses, all administered intramuscularly in the deltoid region, 1 mL each, over a 2-week period (days 0, 3, 7, and 14).
- For persons with immunosuppression, the recommended postexposure prophylaxis series includes five doses of vaccine administered on days 0, 3, 7, 14, and 30.
- Use of both active and passive immunization is strongly recommended for proper treatment.

Thanks for sharing. I have 2 comments :
1- For some reasons, we can not receive the brain biopsy. We do real-time PCR on nuchal skin
2- For post exposition vaccination, there’s the Zagreb protocol :
2 J0 – 1 J7 – 1 J21
Thanks for sharing. I have 2 comments :
1- We also do molecular diagnostic by real time PCR on skin biopsy. Sometimes it’s very difficult to get the brain biopsy.
2- For post-exposition vaccination, there is the Zagreb protocole :
2 shots J0 – 1 J7 and 1 J21.
👍
Really helpful. Thanks for sharing.
Thank you sarga with this very important contribution
Thanks you for the easy to understand presentation.
EXCELLENT!!!!!
I EASILY UNDERSTOOD ALL PHENOMENA RELATED TO RABIES VIRUS
THAAAANKS SAGAR ARYAL!!!!
Thank you so much 🙂
Good information bro,good luck.
Thanks 🙂