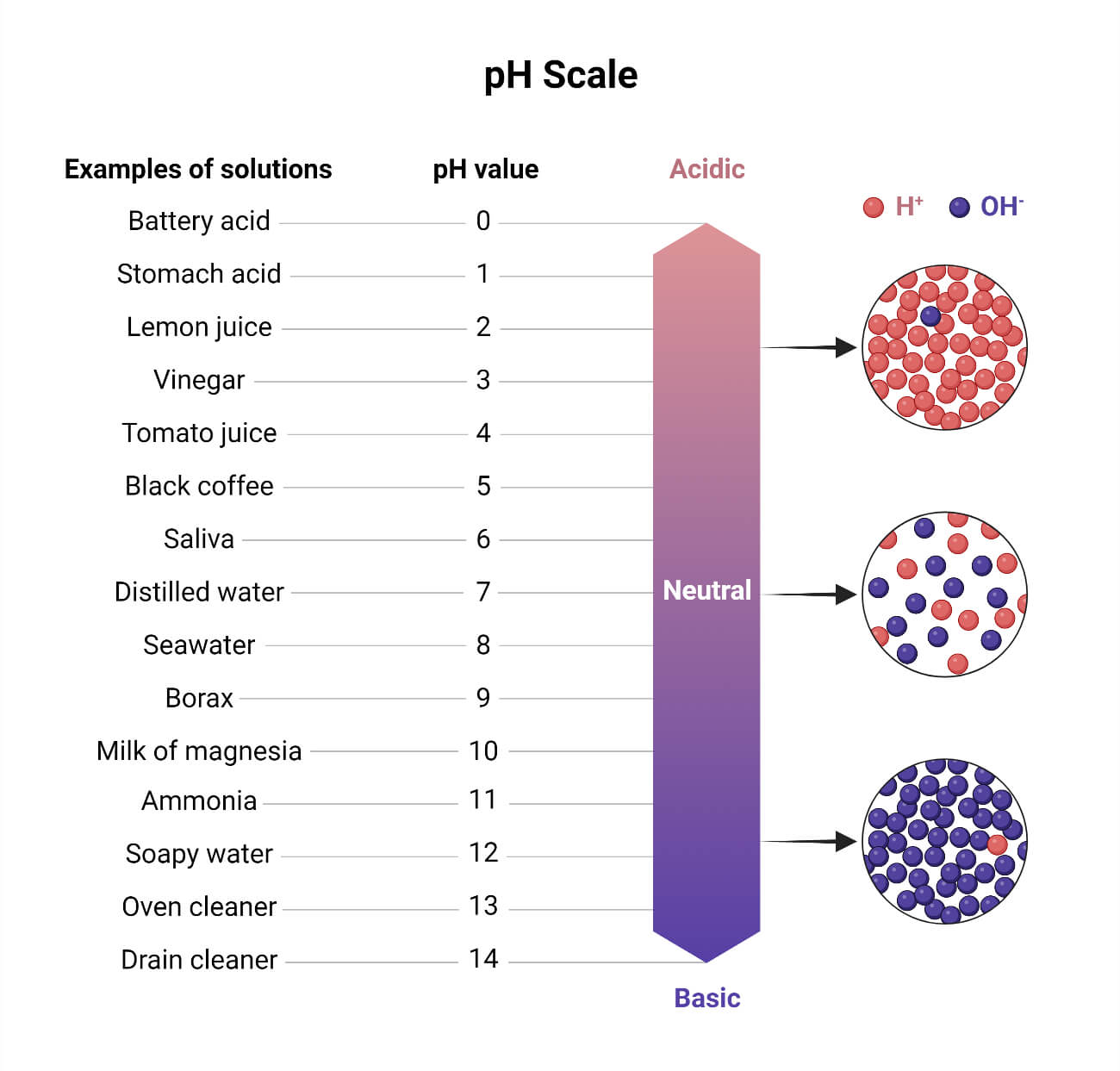
A unit of measure that measures the acidity or alkalinity of a solution using a logarithmic scale with seven as neutral, where lower values are more acidic, and higher ones are more alkaline, is known as pH.
The pH equals negative log10 of the hydrogen ion concentration (c), given in moles per liter (c).
pH = -log10[H+]
where, [H+]= the solution’s hydrogen ion concentration, expressed in moles per liter.
In an aqueous solution, the product of hydrogen ion concentration and hydroxyl ion concentration is constant, and the pH is equal to the negative logarithm of the concentration of hydrogen ions.
A pH meter is a statistical tool that monitors the hydrogen-ion activity in water-based solutions, determining its acidity or alkalinity represented as pH.
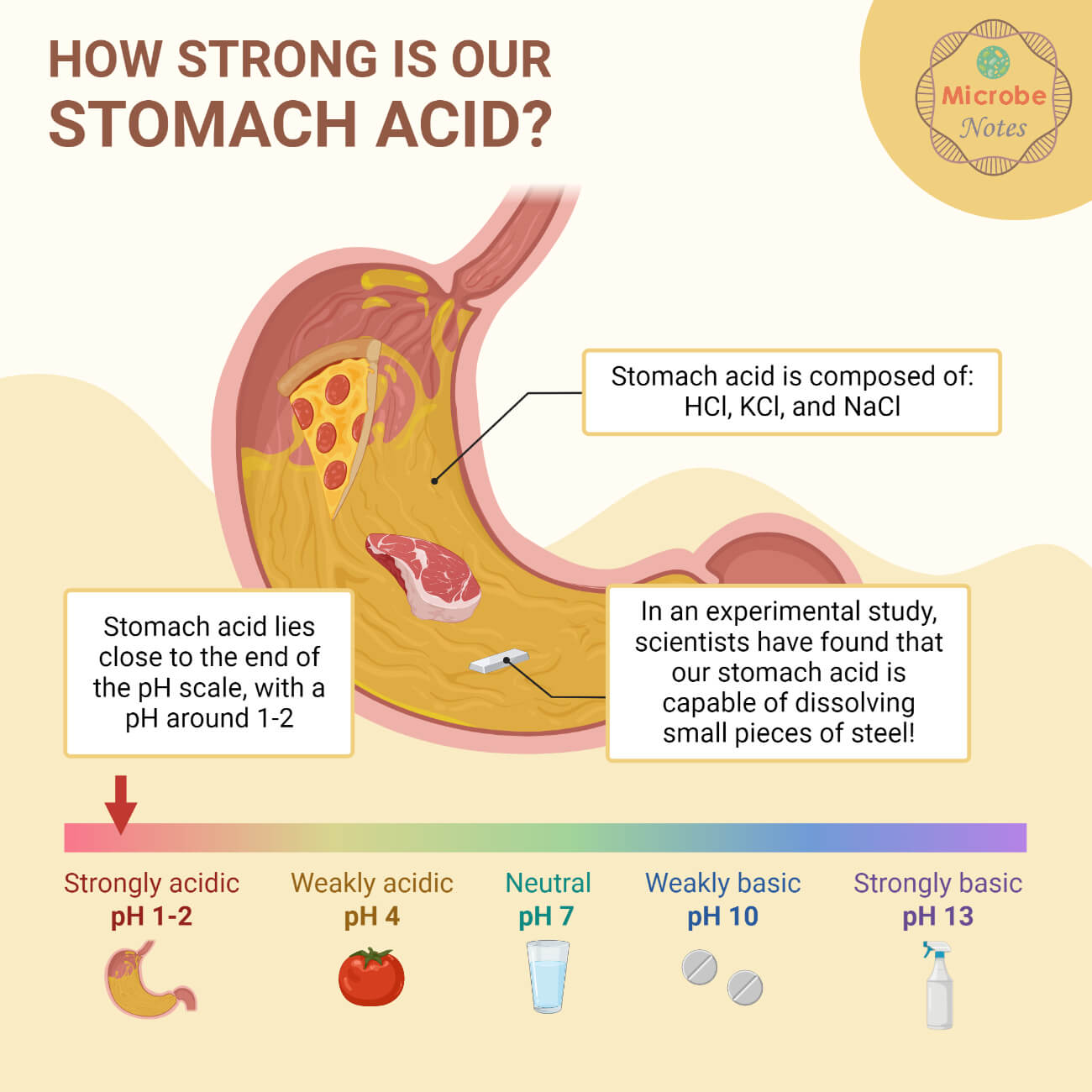
It measures pH on a scale of 0 to 14. The proportion of hydrogen ions (H+) to hydroxyl ions (OH-) determines a substance’s pH value. If the concentration of [H+] exceeds that of [OH-], the substance is acidic. The pH level is below 7. The substance is neutral if the concentration of [H+] and [OH-] are equal. The pH value is 7. The substance is basic if the [H+] concentration is lesser than the [OH-]. The pH level is higher than 7.
The concept of a glass electrode was first introduced in 1909 by Nobel Prize-winning German chemist Fritz Haber (1868–1934), together with his pupil Zygmunt Klemensiewicz (1886–1963). By 1934, American chemist Arnold Beckman had developed the contemporary electronic pH meter (1900–2004).
It is also referred to as a “potentiometric pH meter” since it gauges the difference in electrical potential between a pH electrode and a reference electrode.
pH monitoring is crucial in the areas like the manufacture of specific foods, the culture medium, chemical solutions, soil, quality control, etc. are just a few areas where pH monitoring is crucial.
Interesting Science Videos
Principle of pH Meter
The working principle of the pH meter relies on the ions exchange from the sample solution to the inner solution (pH 7 buffer) of the glass electrode via the glass membrane. A pH meter has a pH probe to conduct the electrical signals to the pH meter, which then displays the pH value of the solution. The pH probe contains two electrodes, namely a sensor electrode and a reference electrode. One is filled with a pH 7 buffer, and the other with saturated potassium chloride solution. The sensor electrode bulb comprises a porous glass membrane coated with metal salts and silica.
When the probe is submerged in a sample solution to measure the pH, hydrogen ions build up around the bulb and take the place of the metal ions. Similarly, some metal ions transfer from the glass (sensor) electrode to the sample solution. Because of low sensitivity to pH changes or complete insensitivity to pH changes, the reference electrode potential offers a constant voltage. This generates some electricity captured by the silver wire by generating potential difference (hydrogen-ion activity). The pH meter converts the voltage of this electric flow into pH value by comparing the generated voltage to the reference electrode.
Increasing the solution’s acidity results in a higher concentration of hydrogen ions, which raises the voltage. The pH measurement on the pH meter decreases due to the increased voltage. Similar to how an increase in alkalinity reduces hydrogen ions, an increase in the concentration of hydroxyl ions also reduces the voltage and raises the pH reading on a pH meter.
Parts of pH meter
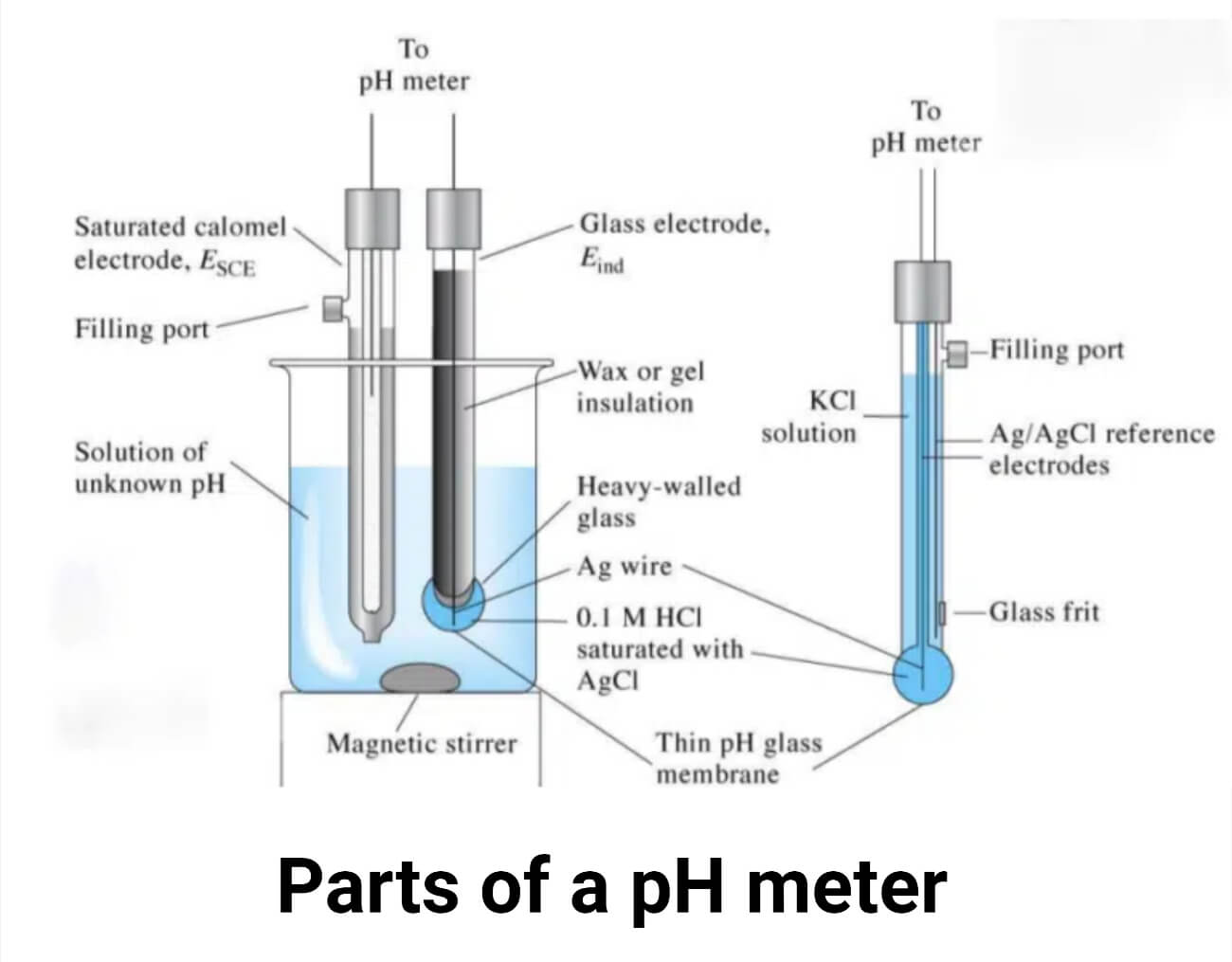
pH meter consists of three basic elements:
- A high input impedance meter
This is the key component that holds the microprocessor that processes extremely small electrode voltages and displays measurements in pH units on display. The microchip reads the pH of the solution, calculates the measurement temperature, and translates the amplifier voltage value.
- The combined electrode
It consists of two electrodes, where the actual measurement takes place. It is the most expensive, sensitive, and consumable component of the meter that needs to be handled carefully. A reference electrode and a measuring electrode or sensor electrode, both submerged in the same solution, make up the combination electrode. The reference electrode must have a defined stable voltage independent of the measured solution to produce a defined pH value.
Reference electrode: A reference electrode is made up of a reference material (such as mercury, mercury chloride, and saturated solution of potassium chloride) submerged in a specific electrolyte which needs to be interacting with the measured solution most frequently through a porous ceramic junction, have a low electrical resistance due to a high ion concentration and adequate stability across a broad temperature range. It has a known and constant potential.
pH glass electrode: It is a glass bulb sensitive to hydrogen ions, and when the relative concentration of hydrogen ions within and outside the bulb changes, so does the millivolt output. It is also known as a sensor electrode or indicator electrode.
- Amplifier
An amplifier, also known as a voltage amplifier, plays a vital role in measuring pH value. The amplifier will increase the accuracy of the pH reading in the same way that a thermometer increases calculations concerning temperature. To precisely measure the amount of acidity, basicity, and neutrality in a solution, this component will ensure that the voltage count is in the pH range of 0–14.
- Thermometer probe
Some pH meters can measure the temperature of the solution being sampled and incorporate that information into the meter reading (the temperature of the solution directly influences pH). This feature is termed “Automatic Temperature Compensation (ATC)”.
pH Meter Operating Procedure
- Let all the samples get to the same temperature since pH readings rely on temperature. It is advised to compensate for temperature if the samples are not at 25 °C. Determine the temperatures of the samples using a thermometer and manually enter them into the meter, or use an ATC probe to communicate the temperatures automatically.
- Uncover the sample beakers and prepare the samples.
- Rinse the pH electrode in the sample beaker after rinsing it with deionized water beforehand. To prevent sample contamination, rinse the electrode with deionized water over a waste beaker. The identical beaker used for sample measurement should never be used to rinse the electrode.
- The electrode should be inserted into the first sample measurement beaker with the electrode tip and junction completely submerged in the sample. The sample should then be stirred moderately and uniformly.
- Set the meter to begin taking a reading.
- Record the pH and temperature of the sample after waiting at least 1 to 2 minutes for a stable reading in the sample.
- If more samples are needed, repeat steps 3 through 6 again. For the most accurate sample measurements, submerge the electrode in each sample to the same depth. After measuring the samples, clean the electrode with deionized water and put it in a pH electrode storage solution.
Types of pH meters
Based on Portability
Pen testers: Pen testers are portable, inexpensive pH meters the size of a pocketbook. The compact form makes them incredibly simple to transport and use while on the road. They are designed with a pH meter, display, and electrode. Pen testers have many uses in the building, hydroponics, food production, and pool or spa care industries.
Handheld meters: Handheld meters often have a more robust build and a slightly larger shape than pen testers. With this design, the electrode is constructed independently of the meter. Depending on your demands for pH measurement, handheld meters typically feature electrodes you may switch out. For medium-to-firm items, for instance, spear-tipped electrodes are utilized. Hand-held meters are designed for usage in the field. Environmental officers use them in field research, aquaculture, agriculture, and water treatment.
Benchtop pH meters: The largest of the three pH meter categories are benchtop meters. They can be put on a wall or a desk. They are often the most accurate pH meters available, which makes them ideal for usage in laboratories and professional settings. Benchtop pH meters are frequently used in laboratories for environmental monitoring, water testing facilities, and food processing facilities.
Based on Usage
Laboratory pH meter: It has a large measuring range, is highly accurate, and is versatile.
Industrial pH meter (online): Its distinctive qualities, which combine analog output, digital intelligence, and upper and lower boundary alarm and control functions, include exceptional stability, steady work, a high level of measurement efficiency, environmental flexibility, and anti-interference capabilities.
Based on advanced level
Economic pH meter
Intelligent pH meter: It has many applications, including water conditioning, aquariums, fish hatcheries, food processing, photography, laboratory, paper industry, etc.
Precision pH meter: It is further categorized as pointer pH meters and digital pH meters.
Based on reading
Analog pH meter
An analog pH meter is the original type of model. A pointer will show the pH level on analog pH meters. The needle will move toward a number representing the pH level after the measuring electrode has been put into the sample. When using an analog pH meter, one must be careful to obtain accurate findings. The little pointer is the reason for this.
Digital pH meter
The development of analog pH meters led to the creation of the digital pH meter. The number printed on a digital pH meter’s measuring device is a clue as to what pH level is being measured. This makes it simpler to obtain accurate results concerning samples. However, the basic operations of analog and digital pH meters are still the same.
Applications of pH meter
- A pH meter is essential for assessing soil in the agricultural sector. A pH meter is required because major crops need an alkaline climate. Additionally, they are utilized to gauge the pH of the soil, which will aid in maximizing returns and yields from the soil.
- Monitoring pH level is essential in water treatment facilities and RO water purifiers.
- Chemical industries use pH meters to neutralize wastewater from the steel, pulp, paper, pharmaceutical, biotech, and petrochemical industries.
- A pH meter determines the pH value of chemical compounds and food products to ensure their quality and safety.
- The food industry specifically uses pH meters in the context of dairy products.
- To determine the type of biological conditions by measuring the pH of biological fluids such as blood, urine, gastric acid, etc.
- Employed in detergent manufacturing.
Advantages of pH Meter
- Well-matched for continuous automatic recording and control of industrial and commercial processes
- Permits rapid and reproducible measurements
- Simple to control and operate.
- Used for both oxidizing and reducing solutions
- Does not affect the solution under examination.
- Suitable for use in colloidal, turbid, and colorful solutions.
- This device gives the most accurate and precise value of pH.
- pH meters are portable, so they can be easily used everywhere while traveling.
Limitations of pH Meter
- pH meters should be regularly cleaned to avoid possible contamination of samples. When exposed to corrosive chemicals, the glass tip of the probe used in pH meters can easily break or get damaged.
- External factors like temperature impact the output readings of the pH meter. Thus, pH meters must be calibrated before use to obtain accurate results unless our results may be distorted.
- Deposits on electrode membranes can affect the processes.
- A special buffer solution is needed to calibrate the pH meters.
Precautions
- pH electrodes are sensitive and fragile, so one should not use them as a glass rod to stir the solution while measuring pH.
- pH meters should be calibrated daily before use with the help of standard buffer solutions.
- pH readings are temperature sensitive, so pH meters shouldn’t be exposed to sunlight.
- All the test tubes and glass apparatus used in measurement should be cleaned with distilled water before use.
- For each new sample, either uses a brand-new fine dropper or glass rod or thoroughly wash the dropper or rod in water between uses.
- All the solutions used in measurement should be freshly prepared.
pH Meter Examples
Laboratory pH meter pH 1120X (Mettler Toledo)
- The pH meter 1120’s unique design enables optimal use of the instrument in various applications.
- For potentially explosive environments, the explosion-proof version 1120-X was specially created for the chemical industry.
- The entire unit, including the non-reflective and scratch-proof glass display panel, is completely chemical resistant.

Water analysis pH meter LAQUAtwin pH-11 (SHISAS TRADING CONCERN PVT. LTD.)
- The sole pocket meter that detects pH in a sample of 0.1 milliliters (or 0.05ml sample with sampling sheet B).
- A special sensor enables the measurement of viscous liquids, solids, and powder samples. Only a few drops of the standard or sample must be applied to the flat sensor.
Gastroesophageal pH meter PH DAY2 (MEDICA)
- The portable device PH DAY2 uses two channels to record the gastroesophageal pH readings.
- Lightweight, portable, and powerful design enable 24-hour recording.
Portable pH meter 913 (Metrohm)
- Portable pH meter with two galvanically isolated pH measurement inputs and built-in battery pack
- Analog pH measurement input for pH electrodes using the Metrohm standard
- Digital pH measurement input for Metrohm’s smart pH electrodes
- Strong, water-tight, and dust-tight housing (IP67) for use in laboratories and harsh outdoor environments
- For easy results and legibility, an LCD color display with backdrop illumination.
References
- https://pharmaguddu.com/ph-meter-principle-calibration-working/
- https://www.pharmaguideline.com/2015/08/principle-and-working-of-pH-probes.html
- https://microbiologie-clinique.com/laboratory-ph-meters.html
- https://electricalfundablog.com/ph-measurement-working-principle-applications/
- https://assets.thermofisher.com/TFS-Assets/CMD/Product-Bulletins/TN-ph-calibration-procedure-for-optimal-measurement-precision-T-PHCAL-EN.pdf
- https://www.instrumentchoice.com.au/news/what-types-of-ph-meters-are-there
- https://pharmabeej.com/types-of-ph-meter/
- https://hyprowira.com/en/blog/variety-types-functions-of-pH-Meters
- https://chrominfo.blogspot.com/2019/04/advantages-and-disadvantages-of-ph-meter.html
- https://www.medicalexpo.com/prod/horiba-scientific/product-104260-863724.html
- https://www.medicalexpo.com/prod/mettler-toledo/product-74754-740618.html
- https://www.medicalexpo.com/prod/medica/product-69252-1071609.html
- https://www.directindustry.com/prod/metrohm/product-15372-1565723.html

Indeed this article really helped me in my research assignment
Thank you so much
the notes are very summarized and brief but they are good
I found this article on pH meters by Microbe Notes to be incredibly informative. The detailed explanation of the principle, parts, procedure, types, and uses of pH meters provides a comprehensive understanding of their functionality. The examples provided further illustrate the practical applications of pH meters in various fields. Microbe Notes consistently delivers well-researched and educational content. Thank you for sharing this valuable resource!
Congratulations 👏👏