OF test is the test to differentiate bacteria into two groups, oxidative bacteria and fermentative bacteria to biochemically characterize and identify them.
Carbohydrates, simply called sugars, are the polyhydroxy aldehydes or ketones that are composed of carbon (C), hydrogen (H), and oxygen (O) atoms combined in an empirical combination of Cx(H2O)y. They are the major source of carbon and energy for every lifeform. Accordingly, different microorganisms utilize different types of carbohydrates in varied manners. This pattern of carbohydrate utilization varies among bacterial genera and even within different species of a single genus. Therefore, studying carbohydrate metabolizing capacity and metabolizing patterns can be used to biochemically characterizes microorganisms into different groups.
Bacteria can catabolize carbohydrates and produce acid either aerobically or anaerobically. The sugar (glucose) if catabolized aerobically i.e. in glycolysis where the oxygen molecule (O2) serves as a terminal electron acceptor, the process is called oxidative metabolism. Similarly, if the sugar is catabolized anaerobically i.e. in the fermentation process where the inorganic ions serve as the terminal electron acceptor, the process is called fermentative metabolism.
In 1953, Hugh and Leifson studied such phenomenon and devised a method to differentiate bacteria into groups; oxidative bacteria (bacteria that can produce acid only in aerobic conditions) and fermentative bacteria (bacteria that can produce acid in both aerobic as well as anaerobic conditions) using a special oxidative-fermentation (OF) medium, called the oxidative-fermentation test (also called the oxidation-fermentation test) or OF test.
Interesting Science Videos
Objectives of OF Test
- To identify if the test bacteria can catabolize sugar and produce acid in aerobic conditions or both conditions or can’t use sugar (i.e. are non-saccharolytic).
- To differentiate bacteria into two groups; oxidative bacteria and fermentative bacteria to biochemically characterize and identify them.
Principle of OF Test
Bacteria can metabolize sugar (glucose) and produce acid either via oxidation (aerobically) or via fermentation (anaerobic) or both. The anaerobic fermentation results in the production of a mixture of different organic acids. This causes a higher concentration of acid in the medium decreasing pH of the medium. This decrease in pH causes the bromothymol blue indicator of the medium to change color from green to yellow.
During aerobic respiration, only a small amount of weak organic acid is produced during glycolysis and the Krebs cycle. There results in a decreased amount of peptone and an increased amount of glucose in the media. This weak acid in the presence of a higher glucose and lower peptone can be detected by the bromothymol blue indicator in the medium. The addition of a dipotassium phosphate buffer further increases the detection of this weak and small amount of acid developing yellow color in the media.
Requirements of OF Test
A. Culture Media
Hugh and Leifson’s medium (also called the OF basal medium) is used in the OF test.
Composition of OF Basal Media in 1000 mL
Peptone (Tryptone)- 2.0 grams
Sodium Chloride- 5.0 grams
Dipotassium Hydrogen Phosphate- 0.3 grams
Bromothymol Blue- 0.03 grams
Agar- 2.0 grams
Final pH- 6.8 ±0.2 at 25°C
(References: OF Basal Medium (himedialabs.com))
In this basal medium 10% (10 grams in 1000 mL medium) carbohydrate of choice mainly glucose, sucrose, dextrose, maltose, or lactose are added.
Preparation of OF Medium
- Measure the appropriate amount of OF basal medium (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company.
- Add 10 grams of the carbohydrate of choice (like glucose) to the solution.
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve completely in water.
- Autoclave the flask or bottle at 121°C and 15 lbs pressure for 15 minutes and let it cool to around 40 – 45°C.
(If carbohydrate was not added earlier, now add 100 mL of filter-sterilized 10% carbohydrate solution.)
- In the sterile test, tubes dispense 5 mL of the medium aseptically. (2 tubes per sample is required)
- Let the media solidify entirely by leaving the tubes in a standing position. (Store in a freeze at 4°C for use up to 4 weeks)
B. Equipment
| Sterile Test-tubes Paraffin Oil | Weighing Machine Autoclave & Incubator | Bunsen burner Syringe Filter | Micropipette Inoculating Wire |
PPE and other general laboratory materials
C. Test Organisms (Fresh culture of sample bacteria)
Glucose fermenter: E. coli ATCC 25922
Glucose oxidizer: Pseudomonas aeruginosa ATCC 27853
Glucose Non-saccharolytic: Alcaligenes faecalis (Moraxella spp.)
Procedure of OF Test
- Using inoculating wire, inoculate two tubes of OF medium by stabbing “halfway to the bottom” (around 2.5 to 3 cm depth).
- In one tube, pour paraffin oil (or mineral oil), making a layer of 1 cm. (This creates an anaerobic condition and makes the tube fit for anaerobic fermentation.)
- Incubate both tubes at 35±20C for about 24 to 48 hours (some late fermenters may require incubation up to 4 days before observing the color change).
- Following the incubation, read the color change in both the tubes and the report.
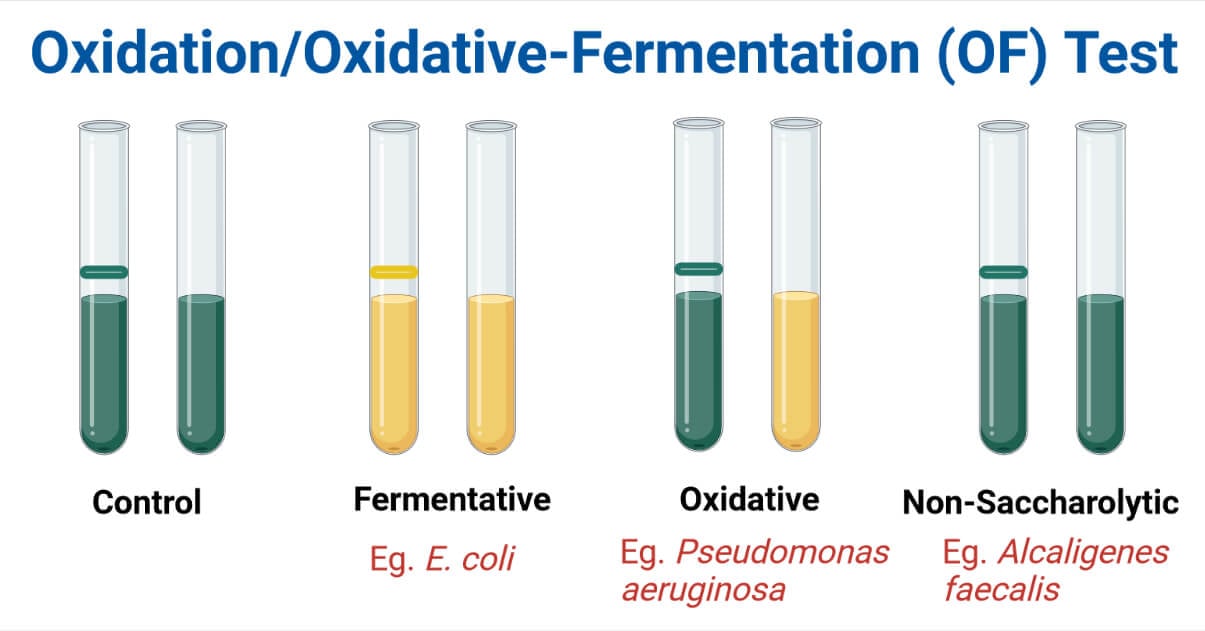
Result and Interpretation of OF Test
| Color of Medium in | Color of Medium in | |
| Tube With Paraffin Oil (Anaerobic Culture/Fermentation) | Tube Without Paraffin Oil (Aerobic Culture/Oxidation) | Results |
| Yellow (Acidic) | Yellow (Acidic) | Fermentative Bacteria |
| Green (Alkaline) | Yellow (Acidic) | Oxidative Bacteria |
| Green (Alkaline) | Green (Alkaline) | Non-Saccharolytic |
Quality Control
E. coli are glucose fermenters and produce yellow color in both open (aerobic) tubes and oil-covered (anaerobic) tubes if glucose or dextrose is added as a carbohydrate.
Pseudomonas aeruginosa is a glucose oxidizer and produces yellow color in an open tube but the color doesn’t change in the oil-covered tube (if glucose or dextrose is added as a carbohydrate).
Alcaligenes faecalis is non-saccharolytic and there is no color change in both open and oil-covered tubes.
Precautions
- Make sure that the carbohydrate solution is sterile before adding it to the OF basal medium.
- Don’t use acidic mineral oils because they can give a false positive result for fermentation.
- While inoculating use sterile inoculating wire and stab about halfway of the medium.
- Properly seal one tube using sterile mineral oil leaving the other tube open.
Applications of OF Test
- Used in the identification of gram-negative bacteria based on their biochemical characteristics
- Used to study the carbohydrate utilizing ability and metabolizing process of the bacteria
Limitations of OF Test
- Need for other tests to confirm the identity of the bacteria
- Some bacteria ferment/oxidize one type of carbohydrate and not the other types, while other types of bacteria utilize other types of carbohydrates. Hence, there is no appropriate media composition.
- OF medium may not be suitable for growing and testing fastidious organisms.
- Some bacteria are late fermenters and may require prolonged incubation of 3 days to 14 days.
- Mineral oil may be acidic, causing the medium’s color to go from green to yellow.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- Carbohydrates: Definition, Formula, Classification, Importance, Examples (toppr.com)
- OF (Oxidation-Fermentation) Test – Procedure, Uses and Interpretation (microbiologyinfo.com)
- Oxidative Fermentative test test: Introduction, Principle, procedure ,results (universe84a.com)
- Oxidative-Fermentative-Test-Protocol.pdf (asm.org)
- Oxidation Fermentation Test: Result, Principle, Procedure (researchtweet.com)
- Oxidation-Fermentation (OF) test: Objective, Principle, Procedure and Result – Online Biology Notes
- OF (Oxidation-Fermentation) Test Principle, Purpose, Procedure (microbiologynote.com)
- Oxidative fermentative (OF) test: Principle, Procedure, Results (microbeonline.com)
- OF Basal Medium (himedialabs.com)
