The oxidase test is a biochemical testing module used to assay the ability of bacteria to synthesize cytochrome c oxidase enzymes.
This test was first introduced by Gordon and McLeod in 1928 to distinguish Neisseria gonorrhoeae from Staphylococcus spp. and Streptococcus spp. Later it was modified by Kovacs and used Kovacs’ oxidase reagent (tetra-methyl-p-phenylenediamine dihydrochloride) for the identification of the cytochrome oxidase enzyme. Again, Gaby and Hadley modified the test and used p-amino dimethylaniline oxalate with α-naphthol as a reagent to detect the cytochrome oxidase enzyme in tube culture.
Cytochrome c oxidase is a large transmembrane protein acting as the terminal enzyme in the electron transport chain of aerobic bacterial and mitochondrial respiration system that catalyzes the final electron transfer from cytochrome c to oxygen molecule. Some bacteria contain this enzyme and have the ability to transfer a terminal electron to molecular oxygen; however, some bacteria lack this enzyme and fail to transfer a terminal electron to molecular oxygen or may use different cytochrome to do the job. Differentiating the bacteria that contain and do not contain cytochrome c oxidase enzyme is very important to characterize bacteria and identify them biochemically.
Interesting Science Videos
Objectives
- To assess the presence of cytochrome c oxidase enzyme within bacterial electron transport chain system
- To biochemically characterize bacteria and helps in their identification
Principle of Oxidase Test
In the presence of molecular oxygen, the cytochrome c oxidase enzyme of bacteria oxidizes phenylenediamine in the colorless reagent to a deep purple to blue-colored compound, indophenol blue. Hence, if the bacteria possess the cytochrome c oxidase, there will be the development of deep purple/blue color, but if the bacteria lack that enzyme, there won’t be any color change.
Requirements for Oxidase Test
Culture Media
While performing the oxidase test following either the disc method or filter paper method or swab method or direct plate method, there is no need for culture media. Bacteria grown in any selective medium (or pure colonies from any media) can be used for the test.
However, for the tube method (Gaby and Hadley method) of the oxidase test, nutrient broth medium (or any standard broth medium with low glucose content) is required. (Here, we will use nutrient broth.)
Composition of Nutrient Broth per 1000 mL
Peptone- 5.00 grams
HM Peptone B (Beef Extract)- 1.50 grams
Yeast Extract- 1.50 grams
Sodium Chloride- 5.00 grams
Final pH 7.4 ±0.2 at 25°C
(References: Nutrient Broth (himedialabs.com))
Preparation of Nutrient Broth
- Measure the appropriate amount of nutrient broth powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company.
- Stir well using a magnetic stirrer or manually and heat to boiling if necessary so that all the components dissolve completely in water.
- Dispense 5 mL of broth in each test tube and loosely put on the screw cap (or use a cotton plug to cover the opening).
- Autoclave the tubes with nutrient broth at 1210C and 15 lbs pressure for 15 minutes and let it cool to room temperature before inoculation.
Reagents
Kovacs’ Oxidase Reagent (for the disc, filter paper, swab, or direct plate method)
1% N, N, N, N-tetramethyl-p-phenylenediamine dihydrochloride
Preparation of Kovacs’ oxidase reagent:
- Dissolve 1.0 grams of N, N, N, N-tetramethyl-p-phenylenediamine dihydrochloride in 100 mL of sterile distilled water and mix well.
Gordon and McLeod Oxidase Reagent (for the disc, filter paper, swab, or direct plate method)
1% dimethyl-p-phenylenediamine dihydrochloride
Preparation of Gordon and McLeod Reagent
- Dissolve 1.0 grams of dimethyl-p-phenylenediamine dihydrochloride in 100 mL of sterile distilled water and mix well.
Gaby-Hadley Reagents (for tube method)
Reagent A (1% α-naphthol)
- Add 1.0 grams of α-naphthol in 100 mL of 98% ethanol.
Reagent B (1% p-amino dimethylaniline oxalate)
- Add 1.0 grams of p-amino dimethylaniline oxalate in 100 mL of distilled water.
Impregnated Oxidase Disc/Test Strip
Equipment
| Petri Plates Whatman no.1 Filter paper (disc or strip) | Weighing Machine Autoclave | Bunsen burner Test Tubes | Dropper Inoculating loop (Cotton Swab) | PPE Other laboratory materials |
Test Bacteria (Sample bacteria (well-isolated colonies))
Positive control: Pseudomonas aeruginosa ATCC 2783
Negative control: E. coli ATCC 25922
Procedure of Oxidase Test
(Gordon and McLeod Oxidase Reagent or Kovacs’ Oxidase Reagent can be used for oxidase test following the filter paper method, swab method, or direct plate method. Kovacs’ oxidase reagent has higher sensitivity than Gordon and McLeod reagent and comparatively gives faster and clearer results. Therefore, Kovacs’ oxidase reagent (tetramethyl-p-phenylenediamine dihydrochloride) is mainly used for the oxidase test. In this procedure, we use Kovacs’ oxidase reagent, but if you have Gordon-McLeod oxidase reagent, you can use it.)
Filter Paper Method
- In a sterile petri plate, place a strip/disc of Whatman no. 1 filter paper. Soak the filter paper with 1% Kovacs’ oxidase reagent and let it dry.
- Using a sterile inoculating loop, pick up a well-isolated colony of test bacteria from a fresh (18 to 24 hours old) culture and make a smear on the reagent-soaked filter paper piece.
- Observe for color change and note the time required for change in color for up to 60 seconds.
Alternatively,
- Pick a well-isolated colony of test bacteria from fresh culture using a sterile inoculating loop and make a smear over Whatman no. 1 filter paper strip/disc.
- Add 1 to 2 drops of Kovacs’ oxidase reagent over the smear.
- Observe for color change and note the time required for change in color for up to 60 seconds.
Swab Method
- Moisten a sterile swab with 1% Kovacs’ oxidase reagent.
- Touch a well-isolated colony from a fresh culture with the swab.
- Observe the development of color in the swab and note the time required for change in color for up to 60 seconds.
Direct Plate Method
- Over well-isolated (pure culture) colonies of test bacteria from fresh culture, add a few drops of Kovacs’ oxidase reagent.
- Tilt the plate and shake it gently so that the colonies get exposed to oxygen.
- Observe for the formation of purple (deep blue) color over the reagent-moistened colonies and note the time required for change in color for up to 60 seconds.
Impregnated Disc/Strip (Oxidase Disc) Method
- Place the impregnated oxidase disc or strip over a clean petri plate (or glass slide) and moisten it with sterile deionized water. (Some discs may not need to be moistened. Look for the manufacturer’s instructions.)
- Using a sterile inoculating loop, pick up a well-isolated colony of test bacteria from fresh culture and make a smear on the oxidase disc/strip.
- Observe for color change and note the time required for change in color for up to 60 seconds.
Tube Method (Gaby-Hadley Oxidase Test)
- Inoculate a nutrient broth medium with sample bacteria and incubate aerobically at 35±2°C for 18 to 24 hours.
- Add 0.2 mL of Gaby-Hadley Reagent A (1% α-naphthol) and add 0.3 mL of Gaby-Hadley Reagent B (1% p-amino dimethylaniline oxalate) and mix well by shaking the medium.
- Observe for color change and note the time required for change in color for up to 3 minutes.
Result and Interpretation of Oxidase Test
Using Kovacs’ oxidase reagent
Positive Test
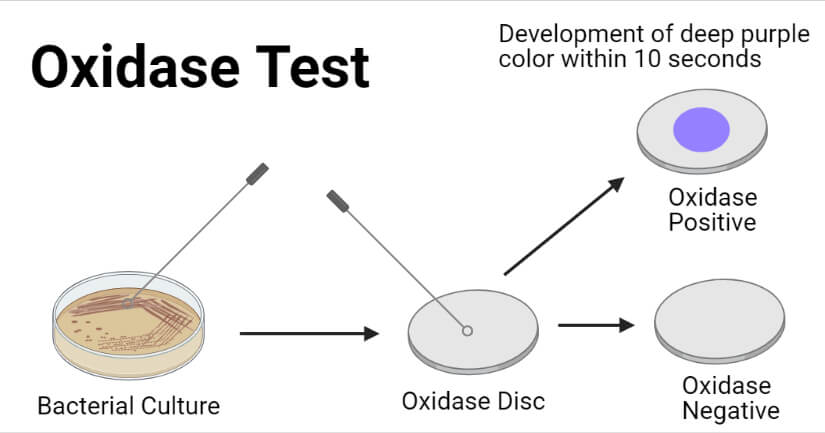
- Development of purple to deep blue color within 10 to 30 seconds indicates a positive oxidase test.
- Development of purple to deep blue color within 30 to 60 seconds indicates a weak oxidase positive reaction or delayed oxidase positive.
Negative Test
- No development of purple to deep blue color within 60 seconds.
- Development of purple to deep blue color after 60 seconds.
Using Gaby-Hadley Reagents (For Tube Method)
Positive Test
- Development of purple to deep blue color within 15 to 30 seconds indicates a positive oxidase test.
- Development of purple to deep blue color within 2 to 3 minutes indicates a weak oxidase positive reaction or delayed oxidase positive.
Negative Test
- No development of purple to deep blue color within 3 minutes.
- Development of purple to deep blue color after 3 minutes.
Oxidase Positive Bacteria
Neisseria gonorrhoeae, Neisseria spp., Pseudomonas aeruginosa, Aeromonas spp., Vibrio spp., Brucella spp., Moraxella spp., Micrococcus spp., Bordetella pertussis, Campylobacter spp., etc.
Oxidase Negative Bacteria
E. coli and all Enterobacteriaceae except Plesiomonas shigelloides, Staphylococcus spp., Streptococcus spp., Pseudomonas maltophilia, Mycoplasma spp., Bordetella parapertussis, Listeria spp., etc.
Variable Oxidase Result Showing Bacteria
Haemophilus spp., Brucella spp., Pasteurella spp.
Quality Control
Positive control: Pseudomonas aeruginosa ATCC 2783 rapidly produce deep blue or purple color within 10 to 30 seconds.
Negative Control: E. coli ATCC 25922 doesn’t result in the formation of deep blue or purple color within 60 seconds.
Precautions during Oxidase Test
- Use fresh culture of bacteria for testing.
- Don’t test bacteria grown on media with dyes (like EMB, MAC medium).
- Don’t test bacteria grown on a glucose-rich culture medium.
- Don’t test strict anaerobes.
- Use freshly made oxidase reagent.
- While storing the oxidase reagent, store it in a dark place at – 200C.
- The sample must be taken from well-isolated colonies. Never perform the direct plate method if the culture is mixed culture. (It is recommended to use cultures from selective media to ensure sample purity.)
- Don’t overflood the plate with an oxidase reagent.
- Never record the result after 60 seconds while using Kovacs reagent and after 3 minutes while using Gaby-Hadley Reagents.
- Record time exactly to differentiate rapid oxidase-positive, delayed oxidase-positive, and oxidase-negative bacteria.
- Do not use nichrome wire loops, as they can give false-positive results.
Applications of Oxidase Test
- To determine the ability of bacteria to synthesize the cytochrome c oxidase enzyme.
- Differentiation of Neisseria spp. (oxidase positive cocci) from Staphylococcus spp. and Streptococcus spp.(oxidase negative cocci)
- Differentiation of Enterobacteriaceae from other Gram-negative bacilli.
- Differentiation of Pseudomonas aeruginosa from Enterobacteriaceae.
Limitations of Oxidase Test
- It doesn’t give a confirmatory result and needs further biochemical tests for complete identification.
- The reagents must be prepared freshly. The reagents have been shown to auto-oxidize and are photosensitive; hence it is recommended to prepare reagents daily.
- If the direct plate method is performed, the reagent-soaked colonies will be quickly nonviable, so they need immediate subculture.
- Bacteria grown on glucose-rich media shows false negative results.
- Bacteria from older cultures may give false negative results.
- Commonly used Nichrome or iron inoculating loop may give a false-positive result. Hence, you need either a plastic loop or an expensive platinum loop.
- Need pure culture or well-isolated colonies for testing.
- Need precise recording of time taken for the development of color change.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- MacFaddin JF, editor. Biochemical Tests for Identification of Medical Bacteria. 3rd ed. Philadelphia:Lippincott Williams and Wilkins; 2000. p. 363-7
- Michel H, Behr J, Harrenga A, Kannt A. Cytochrome c oxidase: structure and spectroscopy. Annu Rev Biophys Biomol Struct. 1998;27:329-56. doi: 10.1146/annurev.biophys.27.1.329. PMID: 9646871.
- Chavan, Dharmappa & Khatoon, Halima & Anokhe, Archana & Kalia, Vinay. (2022). Oxidase test: A biochemical method in bacterial identification. (PDF) Oxidase test: A biochemical method in bacterial identification (researchgate.net)
- Pseudomonas biochemical tests – BiochemGems
- Oxidase positive bacteria – BiochemGems
- Oxidase Test: Principle, Procedure, Results • Microbe Online
- 27: Oxidase Test – Biology LibreTexts
- Oxidase Test: Purpose, Principle, Method, Interpretations, Limitations (medicallabscientist.org)
- What is Oxidase Test ? Principle, Composition, Interpretation of Results – Laboratoryinfo.com
- oxidase-test-protocol-3229.pdf (asm.org)
- Oxidase test: Principle, Procedure, Result interpretation and Precautions – Online Biology Notes
- Oxidase test: Principle , procedure, Result interpretation and various (universe84a.com)
- Gaby-Hadley Reagent B (himedialabs.com)
- Gaby-Hadley Reagent A (himedialabs.com)

Very comprehensive information. Great job!
Hi m sarah ,i found very ease after reading this ,thnku so much but when we use colony of neisseria ,does it also shows purple clr in oxidase test
If add discussion and procedure so become best.