Interesting Science Videos
What is NMR?
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei.
It is a spectroscopy technique that is based on the absorption of electromagnetic radiation in the radiofrequency region 4 to 900 MHz by nuclei of the atoms.
Over the past fifty years, NMR has become the preeminent technique for determining the structure of organic compounds.
Of all the spectroscopic methods, it is the only one for which a complete analysis and interpretation of the entire spectrum is normally expected.
Principle of Nuclear Magnetic Resonance (NMR) Spectroscopy
- The principle behind NMR is that many nuclei have spin and all nuclei are electrically charged. If an external magnetic field is applied, an energy transfer is possible between the base energy to a higher energy level (generally a single energy gap).
- The energy transfer takes place at a wavelength that corresponds to radio frequencies and when the spin returns to its base level, energy is emitted at the same frequency.
- The signal that matches this transfer is measured in many ways and processed in order to yield an NMR spectrum for the nucleus concerned.
Working of Nuclear Magnetic Resonance (NMR) Spectroscopy
- The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers.
- The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups.
- As the fields are unique or highly characteristic to individual compounds, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.
- Besides identification, NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules.
- The most common types of NMR are proton and carbon-13 NMR spectroscopy, but it is applicable to any kind of sample that contains nuclei possessing spin.
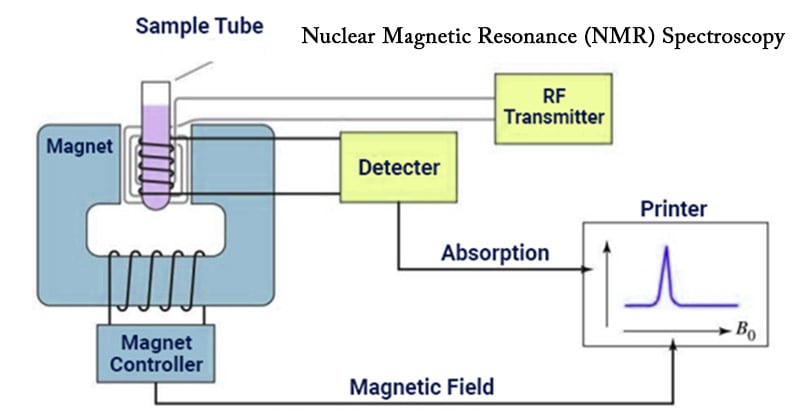
Instrumentation of Nuclear Magnetic Resonance (NMR) Spectroscopy
- Sample holder
Glass tube with 8.5 cm long, 0.3 cm in diameter.
- Permanent magnet
It provides a homogeneous magnetic field at 60-100 MHZ
- Magnetic coils
These coils induce a magnetic field when current flows through them
- Sweep generator
To produce an equal amount of magnetic field pass through the sample
- Radio frequency transmitter
A radio transmitter coil transmitter that produces a short powerful pulse of radio waves
- Radio frequency receiver
A radio receiver coil that detects radio frequencies emitted as nuclei relax to a lower energy level
- Read out systems
A computer that analyses and records the data.
Applications of Nuclear Magnetic Resonance (NMR) Spectroscopy
Spectroscopy is the study of the interaction of electromagnetic radiation with matter. NMR spectroscopy is the use of the NMR phenomenon to study the physical, chemical, and biological properties of matter.
- It is an analytical chemistry technique used in quality control.
- It is used in research for determining the content and purity of a sample as well as its molecular structure. For example, NMR can quantitatively analyze mixtures containing known compounds.
- NMR spectroscopy is routinely used by chemists to study chemical structure using simple one-dimensional techniques. Two-dimensional techniques are used to determine the structure of more complicated molecules.
- These techniques are replacing x-ray crystallography for the determination of protein structure.
- Time domain NMR spectroscopy techniques are used to probe molecular dynamics in solution.
- Solid state NMR spectroscopy is used to determine the molecular structure of solids.
- Other scientists have developed NMR methods-of measuring diffusion coefficients.
References
- http://chem.ch.huji.ac.il/nmr/whatisnmr/whatisnmr.html
- https://www.slideshare.net/solairajananant/nmr-spectroscopy-13887430
- https://www.ias.ac.in/article/fulltext/reso/009/01/0034-0049
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/nmr/nmr1.htm
- https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy

A good website It helps me to much .
very interested and easy to understand
It is great.. Its gives me alot of information.. Its very clear and easy to understand and you can easily clear your concepts.. Thankyou..
Well understood
I enjoyed reading
I really enjoyed reading.
Well understood. Thanks
Hi Eve,
Thank you so much for liking our website and enjoying reading our posts.
Easy learning
It is very easy to understand . thank you