In the anaerobic respiration process, bacteria derive their required oxygen from nitrate (NO3–) or can use NO3– as a terminal electron acceptor. In either of these processes, nitrate is first reduced to nitrite (NO2–) and subsequently to other end products like molecular nitrogen gas (N2), ammonia (NH3), hydroxylamine, etc., or may not further reduce, based on the metabolism process of the bacterium or based on the enzymes present in the bacterium. Such bacteria are called nitrate-reducing bacteria (denitrifying bacteria). Nitrate-reducing bacteria play important roles in soil microbiology and maintaining ecology by recycling nitrogen. These bacteria produce nitrate reductase enzymes that reduce nitrate into nitrite.
Several clinically important bacteria also reduce nitrate. And identification of nitrate-reducing ability plays an important role in identifying several bacterial species. The laboratory biochemical test to determine the ability of bacteria to reduce the nitrate into nitrite is called the nitrate reduction test. In the laboratory, the nitrate-reducing ability of bacteria is tested by culturing bacteria in culture media with a nitrate compound and adding an acid solution of sulfanilic acid and alpha-naphthol.
Interesting Science Videos
Objectives
- To determine the nitrate-reducing ability (production of nitrate reductase enzyme production) of the test bacteria.
- To identify the test bacteria based on biochemical profile.
Principle of Nitrate Reduction Test
Nitrate-reducing organisms produce nitrate reductase enzyme, which reduces the nitrate into nitrite. Thus formed nitrite reacts with acetic acid and forms nitrous acid. The nitrous acid is diazotized with the sulfanilic acid to form a colorless diazonium salt (diazotized sulfanilic acid). The colorless nitrite-sulfanilic acid, when combined with the dimethyl-naphthylamine (α-naphthol), a water-soluble red-colored azo dye (p-Sulfobenzene-azo-naphthylamine) is formed.
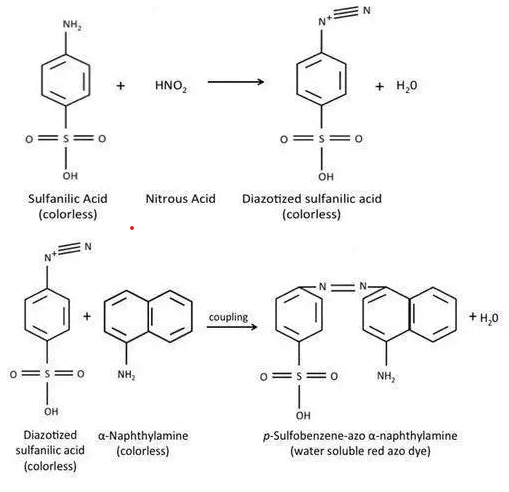
Some organisms can further reduce nitrite into other nitrogen compounds. In this case, there is no formation of red color although the nitrate compound is reduced by the bacteria. Similarly, if there is no nitrate reduction, then also, there is no formation of the red color. To differentiate these two cases, zinc dust is added to detect unreduced nitrate.
The added zinc reduces nitrate into nitrite and results in the formation of red-colored azo dye.
Requirements
Culture Media
Nitrate broth is used to test the bacteria’s ability to reduce nitrate.
Composition:
Peptone 20 grams
(If the test bacteria are fastidious, use 25 grams of heart infusion broth powder in place of peptone)
Potassium nitrate 2 grams
Distilled water 1000 mL
Preparation:
Mix the above-mentioned components in labeled proportion (while using ready-made agar, measure and mix with distilled water in exact quantity as directed by the manufacturer) and shake well to completely dissolve the mixture.
Transfer 4 mL of the medium into a 16 × 125 mm test tube (or more medium based on the volume of the test-tube or the amount which is enough to sink the Durham tube). Tighten the screw cap (or plug the tube with a cotton plug) and autoclave it at 121°C and 15 lbs pressure for 15 minutes.
(Note: the composition may vary based on manufacturing companies. The above-mentioned composition is taken from Leber, Amy L., editor-in-chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]. )
Chemicals/Reagents
0.8% sulfanilic acid as reagent A. 0.5% -napthol as reagent B and pure Zinc metal dust are required.
Preparation of 0.8% sulfanilic acid (Regent A)
Sunfanilic acid 0.8 grams
Distilled water 70 mL
Glacial acetic acid 30 mL
Mix the sulfanilic acid with distilled water and heat it to dissolve completely. Cool the mixture then add acetic acid slowly. (Storing at 2 – 8°C, you can store for up to 3 months)
Preparation of 0.5% -naphthol (Reagent B)
-naphthol (N, N-dimethyl- α-napthylamine) 0.5 grams
Distilled water 70 mL
Glacial acetic acid 30 mL
Mix the glacial acetic acid with distilled water and slowly add the -napthol and mix completely by shaking. (Storing at 2 – 8°C, you can store for up to 3 months)
Equipment
| Test tubes Dropper PPE and other general laboratory materials | Durham tubes Autoclave | Bunsen burner Test-tube holder | Micropipette Inoculating loop |
Nitrate disk (only for the disk method of nitrate reduction test of anaerobic bacteria)
Test organism (Sample bacteria)
Positive controls: E. coli ATCC 25922 as nitrate positive, gas negative
P. aeruginosa ATCC 27853 as nitrate positive, gas positive
Negative control: Acinetobacter baumannii ATCC 19606 as nitrate negative
Procedure of Nitrate Reduction Test
For nitrate reduction tests, three methods, the tube method, the disk method, and the rapid method, are commonly used. Among these methods, the tube method is the most frequently used testing method.
Tube Method
- Autoclave test tubes with nitrate broth and invert Durham’s tube and let them cool to room temperature.
- In a tube, inoculate the test (sample) bacteria from an isolated colony of fresh (24 hours old) culture using an inoculating loop (or drop 2/3 drops of broth containing an overnight culture of the test organism).
- Incubate the tube at an appropriate temperature for the required time period.
* Glucose non-fermenting, Gram-negative bacilli at 25 to 30°C for 24 hours to 5 days.
* Other bacteria at 35 ±2°C for 24 hours to 5 days.
* Campylobacter spp. at 35 ±2°C for at least 3 days at anaerobic or microaerobic conditions.
- After 24 hours, look for visible growth and gas bubbles inside the Durham tube. If there is no gas and no visible growth, incubate the tube for the next 24 hours (or more based on test bacteria).
- If gas is present in the Durham tube in the culture of glucose non-fermenting bacteria, report the test as positive for nitrate reduction and gas production.
- If gas is not present in the Durham tube or if the test bacterium is a glucose fermenter, transfer 0.5 mL of well-mixed culture into another clean (need not to be sterile) test tube.
- Add 3 drops of reagent A and mix well by shaking gently.
- Add 3 drops of reagent B and mix well by shaking gently.
- Observe for the development of red color within 2 minutes.
- If no red color is developed, then add a small amount of zinc dust and observe for the development of the red color within 10 minutes.
- If there is no gas formation and no development of red color after the addition of both reagents A and B, reincubate the tubes and test accordingly after 48 hours and on the 5th day.
Disk Method
Used only for anaerobic organisms.
- On a fresh (24 hours/overnight old) culture of the test organism, place a nitrate disk in the area with heavy growth and incubate anaerobically for 24 to 48 hours.
- Place the nitrate disk on a clean glass slide or petri plate (need not be sterile).
- Add 1 drop of reagent A.
- Add 1 drop of the regent B.
- Observe for the development of red color within 2 minutes.
- If no red color is developed, then add a small amount of zinc dust and observe for the development of the red color within 5 minutes.
Rapid Method
May not be as effective as the tube method, but can be used for quick results if the organism is supposed to be a quick reducer of nitrate and multiply rapidly (have a very short generation time).
- Add 0.5 mL of nitrate broth in a clean test tube, autoclave it for 15 minutes at 15 lbs pressure and 121°C, and let it cool to room temperature.
- Inoculate the tube with a heavy inoculum of fresh bacterial culture.
- Incubate at 35°C for 2 hours.
- Add 2 drops of reagent A and 2 drops of reagent B and mix well.
- Observe for the development of red color within 2 minutes.
- If no red color is developed, add a small amount of zinc dust and observe for the development of the red color within 5 minutes.
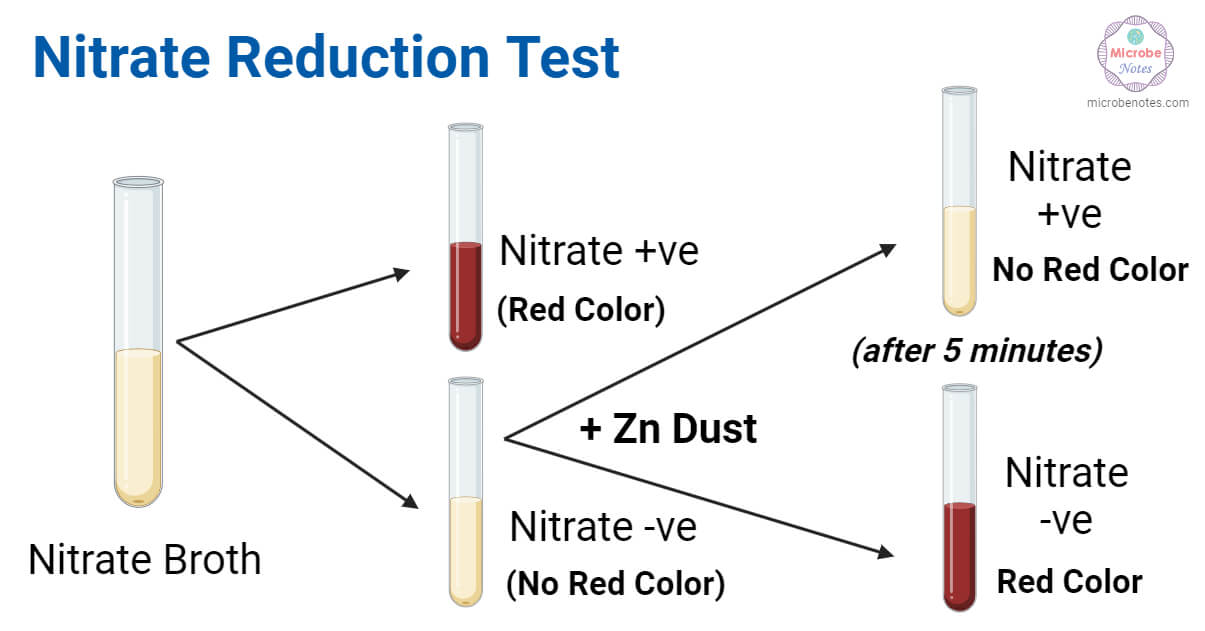
Results and Interpretation of Nitrate Reduction Test
- Formation of gas bubbles (even a single bubble) in the culture of glucose non-fermenting bacteria = nitrate reduction positive, gas positive
- Formation of red color after the addition of reagents and presence of gas in Durham tube = nitrate reduction positive, gas positive
- Formation of red color after the addition of reagents and absence of gas in Durham tube = nitrate reduction positive, gas negative
- No red color formation after the addition of reagents A and B, and also, no red color formation after the addition of zinc dust = nitrate reduction positive (nitrate is reduced to nitrite, and nitrite is reduced to other nitrogen compounds, there is nothing left to turn red).
- No red color formation after the addition of reagents A and B, but the formation of red color after the addition of zinc dust = nitrate reduction negative (no nitrate has been reduced before adding zinc. Upon adding zinc, the untouched nitrate reduces to nitrite and produces the red color).
Quality Control
For checking the quality of the medium and reagents, we can use positive and negative control organisms as mentioned earlier. As for the test organism, label test tubes as “positive control, gas positive”, “positive control, gas negative”, and “negative control” and inoculate the tubes with P. aeruginosa ATCC 27853, E. coli ATCC 25922, and Acinetobacter baumannii ATCC 19606 respectively. Follow all the procedures mentioned above and read the final result after the addition of reagents and zinc dust.
P. aeruginosa ATCC 27853 must give nitrate-positive (red color formation) and gas-positive results.
E. coli ATCC 25922 must give nitrate-positive (red color formation) and gas-negative results.
Acinetobacter baumannii ATCC 19606 must give nitrate negative result (no red color formation).
Bacteria Showing Positive Result
E. coli, Salmonella typhimurium, Enterobacter spp., Bacillus cereus, Mycobacterium tuberculosis, Citrobacter spp., Pseudomonas spp., Klebsiella spp., Proteus spp., S. aureus, etc.
Bacteria Showing Negative Result
Acinetobacter spp., Streptococcus spp., Mycobacterium bovis, M. africanum, etc.
Precautions
- Sterilize the medium properly before use, sterilize the working area, work in a sterile zone, wear proper PPE, and follow laboratory safety rules.
- Be careful while using -napthol as it is carcinogenic.
- Use the appropriate amount of medium in a test tube based on the size of the available test tube and Durham tube. Durham tube must be completely submerged in the broth.
- There must not be any gas bubbles in the Durham tubes before the test.
- Don’t add too much zinc dust. It must not exceed the amount that adheres to the end of the applicator stick (like a toothpick)
Applications of Nitrate Reduction Test
- Understanding the biochemical characteristics of bacteria to identify them phenotypically.
- To differentiate Moraxella catarrhalis from Neisseria spp. and Kingella spp. from Neisseria spp. (N. gonorrhoeae and K. denitrificans)
- Confirmation and identification of Enterobacterales.
- Differentiating Mycobacterium spp.
- Differentiating and identifying Corynebacterium spp.
Limitations of Nitrate Reduction Test
- It is not enough for the identification of bacterial species. It is only a part of the biochemical test; hence other tests are required for complete identification.
- Need of special medium containing nitrate (such as nitrate broth) but free from even small amount of nitrite.
- Difficult to notice the growth of organisms in the nitrate broth.
- Require verification by adding zinc dust to prevent reporting of false negative results if nitrite is further reduced to other nitrogen compounds.
- High chance of a false positive result if the zinc dust is added in a higher quantity.
- Need to know if the organism is a glucose fermenter with gas production or not before the nitrate reduction test and reporting.
- As it is a culture-based method, requires at least 24 hours to 5 days before reporting negative.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- Rosier BT, Moya-Gonzalvez EM, Corell-Escuin P, Mira A. Isolation and Characterization of Nitrate-Reducing Bacteria as Potential Probiotics for Oral and Systemic Health. Front Microbiol. 2020 Sep 15;11:555465. doi: 10.3389/fmicb.2020.555465. PMID: 33042063; PMCID: PMC7522554.
- Cowan & Steel’s Manual for identification of Medical Bacteria. Editors: G.I. Barron & R.K. Felthani, 3rd ed 1993, Publisher Cambridge University Press.
- Goh KS, Rastogi N. Simple and rapid method for detection of nitrate reductase activity of Mycobacterium tuberculosis and Mycobacterium canettii grown in the Bactec MGIT960 system. J Microbiol Methods. 2010 May;81(2):208-10. doi: 10.1016/j.mimet.2010.03.005. Epub 2010 Mar 15. PMID: 20298726.
- Nitrate Reduction Test – Principle, Procedure, Uses and Interpretation (microbiologyinfo.com)
- Nitrate Reduction Test: Principle, Procedure, Results • Microbe Online
- Nitrate Reduction Test Procedure, Principle, Result (microbiologynote.com)
- Nitrate Reduction – Biology LibreTexts
- Nitrate Reduction Test – Laboratoryinfo.com
- Nitrate reduction test: Objective, Principle, Procedure and Result – Online Biology Notes
- Nitrate reduction test Principle, Procedure, Result (medicallabtechnology.com)
- Nitrate Reduction Test: Objective, Principle, Procedure And Result Interpretation – BIOCHEMINSIDER
- Nitrate Reduction Test: Introduction, Principle, Procedure, result (universe84a.com)

Hello Really sorry to correct you but the red color after addition of Zinc Dust states that the Nitrate is not reduced to Nitrite and hence no nitrate reductase activity was present so the test will be negative in that case
Dear Neha,
Thanks for the correction. It should have been as follows:
No red color formation after the addition of reagents A and B, but red color formation after the addition of zinc dust = nitrate reduction positive (nitrate is reduced to nitrite, and nitrite is reduced to other nitrogen compounds)
I think Interp #4 and #5 might need to be revised to:
4. No red color formation after the addition of reagents A and B, *AND NO* red color formation after the addition of zinc dust = nitrate reduction positive (all nitrate has been reduced to nitrite, and all nitrite has been reduced to other nitrogen compounds. There is nothing left to turn red)
5. No red color formation after the addition of reagents A and B but the formation of red color after the addition of zinc dust = nitrate reduction negative (no nitrate has been reduced before adding zinc. Upon adding zinc, the untouched nitrate reduces to nitrite and produces the red color)
Hi Cherry,
Thank you so much for your correction. I have updated the results section as well as the figure. Thank you once again.
Hello sir
You are doing great work .
I m M.Sc Microbiology P.G.D.M.L.T.
I am about to start my own Clinical laboratory in Maharashtra.I want information regarding clinical sample test like urine culture, pus culture,throat culture etc.Can you plz provide me the same with procedure and Standard operating procedures.Kindly do needful.
Contact me
WhatsApp 9028960990
E-mailed:abmajidsir@gmail.com