Nipah virus (NiV) is a zoonotic bat-borne pathogen belonging to the paramyxoviruses.
It was first identified in Malaysia in 1998. However, no cases have been detected in Malaysia since 1999.
The virus is responsible for several outbreaks in the South and Southeast Asian regions.
Pteropus fruit bats are the main reservoir for the pathogen from which spillover can lead to diseases in humans and pigs.
The Malaysia-Singapore outbreak of the virus was associated with contact with pigs whereas the Indo-Bangladesh outbreak was associated with the consumption of raw date palm sap contaminated by fruit bats.
The virus causes severe neurological and respiratory disease that is highly infectious and transmits via infected animals and other infected people.
Due to the high fatality, transmission, and unavailability of effective treatment and vaccine against the virus, it is considered a Biosafety level 4 (BSL 4) pathogen.
Interesting Science Videos
Structure of Nipah Virus (NiV)
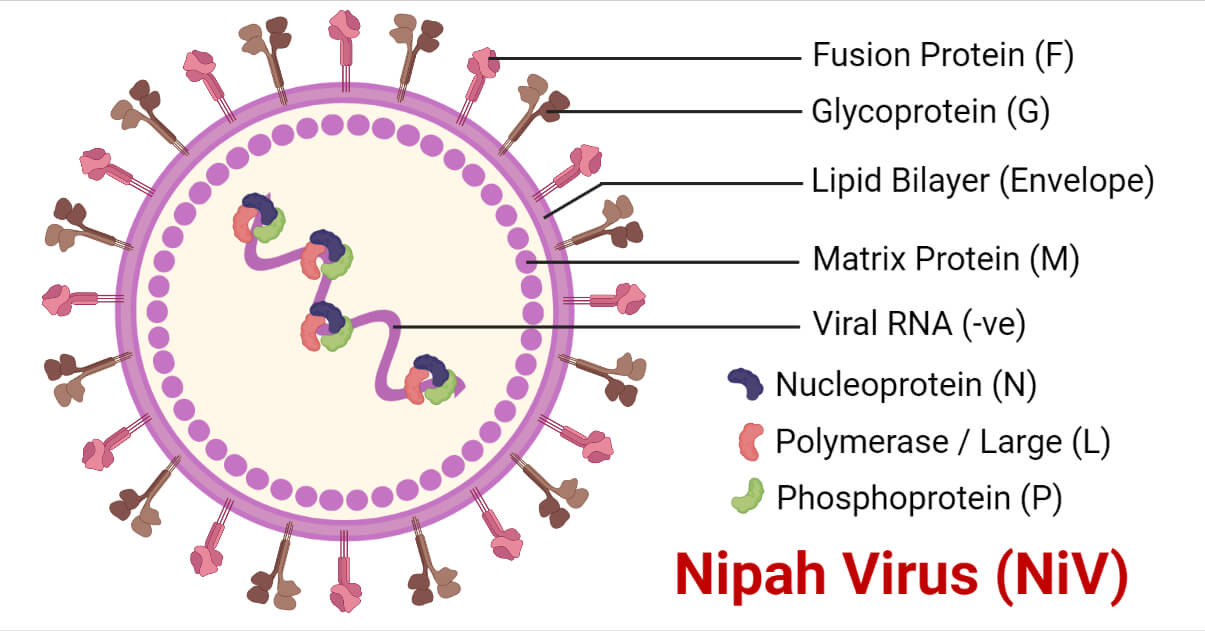
- The Nipah virus is an enveloped virus belonging to the family Paramyxoviridae.
- It consists of a negative-sense, single-stranded, and non-segmented RNA genome with helical nucleocapsid.
- The nucleocapsid, phosphoprotein, and long polymerase constitute the viral ribonucleoprotein.
- The nucleocapsid is surrounded by the matrix protein embedded with fusion proteins and glycoproteins that protrude as spikes, responsible for cellular attachment and host cell entry.
- On average, the NiV are larger than the typical paramyxoviruses with sizes ranging from 40 to 1900nm.
- The virus also differs from other paramyxoviruses in having the reticular cytoplasmic inclusions close to the endoplasmic reticulum.
- The virus has a close similarity to the Hendra virus (HeV) with only minor ultrastructural differences and also shows significant cross-reactivity on various serological tests.
- The two strains; Malaysian (MY) and Bangladesh (BD) strains have approximately 92% sequence similarity but show differences in their pathogenicity and transmission.
Genome structure of Nipah Virus (NiV)
- The genome of the NiV consists of a negative-sense, single-stranded, and non-segmented RNA of approximately 18.2 kbp genome size.
- It codes for six structural proteins; nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), attachment glycoprotein (G), and the large protein or RNA polymerase protein (L).
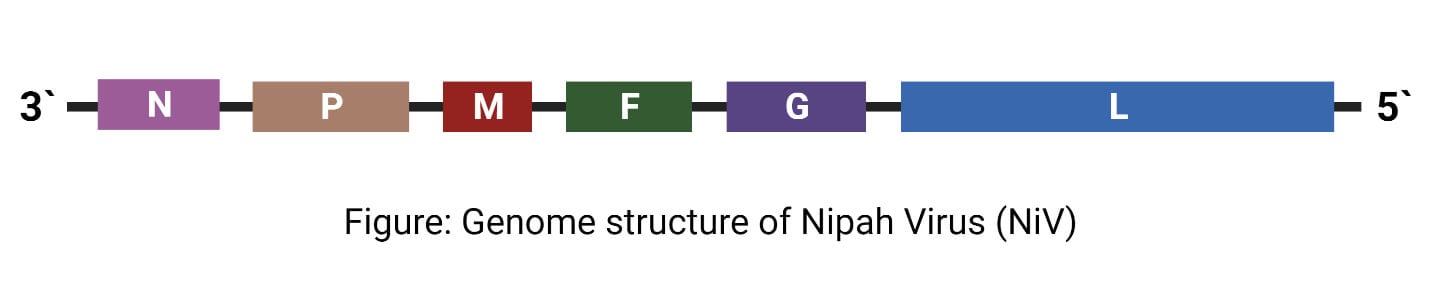
- The P gene also encodes three nonstructural proteins by RNA editing (V and W proteins) or an alternate open reading frame (C protein).
Epidemiology of Nipah Virus (NiV)
- The first outbreak of the Nipah virus was reported in Malaysia-Singapore in 1998-1999.
- From September 1998 to June 1999, 94 patients reporting close contact with the swine population were diagnosed with severe viral encephalitis that showed the direct transmission of the virus from the pigs to humans.
- In Singapore and Malaysia, 246 patients were reported with febrile encephalitis due to NiV between 1998 and 1999. The human mortality rate was approximately 40%.
- The second outbreak was reported in the Meherpur district of Bangladesh and Siliguri city of West Bengal, India in 2001, in a geographically non-contiguous area.
- Overall, 9 outbreaks of the NiV were reported till the year 2010 in Bangladesh.
- Another outbreak in Bangladesh in the year 2011 reported a total of 15 deaths due to the Nipah virus infection.
- Epidemiological investigations showed the circulation of the NiV virus in Asia, Africa, and the South Pacific Ocean causing sporadic outbreaks through human-to-human and zoonotic transmissions.
- It has resulted in the deaths of hundreds of humans during the past two decades and has imposed a huge threat to humans as well as domestic animals.
Transmission of Nipah Virus (NiV)
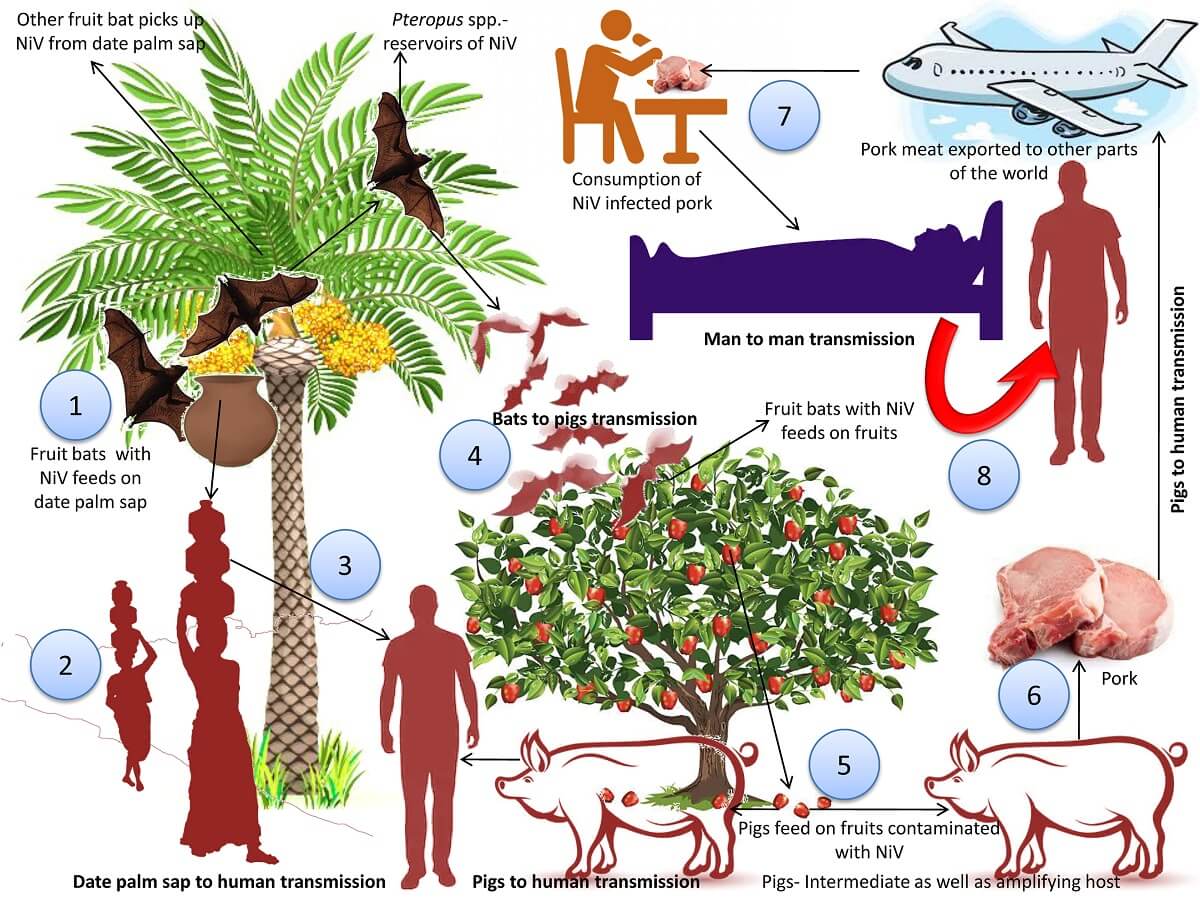
The NiV can transmit to people from:
- Direct contact with infected animals like bats or pigs or their body fluids such as blood, urine, saliva, etc.
- Consumption of contaminated food products that have been contaminated by infected animals (such as the palm sap or fruits contaminated by the infected fruit bats)
- Close contact with NiV infected person or their body fluid (such as nasal or respiratory droplets, urine, or blood)
During the first Malaysia-Singapore outbreak, human infections resulted from direct contact with infected pigs or their body fluids.
The most likely source of infection during the subsequent outbreaks in Bangladesh and India was found to occur through the consumption of fruits and fruit products (such as raw date palm juice) contaminated with the saliva from the infected fruit bats.
During the later outbreaks of the NiV virus in Bangladesh and India, the NiV was observed to spread directly from human to human through close contact and body fluids and secretions.
Human-to-human transmissions were also reported in many of the family members and caregivers of the infected patients.
Replication of Nipah Virus (NiV)
- Attachment/Adsorption
The virus attaches to its host cell through the viral glycoprotein G with the B2 receptor followed by the fusion of the viral membrane with the host membrane.
- Penetration and Biosynthesis
The negative RNA genome serves as a template for the transcription of viral mRNA that is further translated into viral proteins. The vRNA also serves as a template for the synthesis of cRNA(+) which further serves as the template for the synthesis of vRNA. The viral proteins also function in interferon signaling. The F proteins are also endocytosed and matured in the cells.
- Assembly
The assembly is done primarily by the M protein, and N, P, C, M, F, and G, are incorporated in the virions.
- Release
The virions bud through the host membrane containing the G proteins on its surface and get released from the cell.
Pathogenesis of Nipah Virus (NiV)
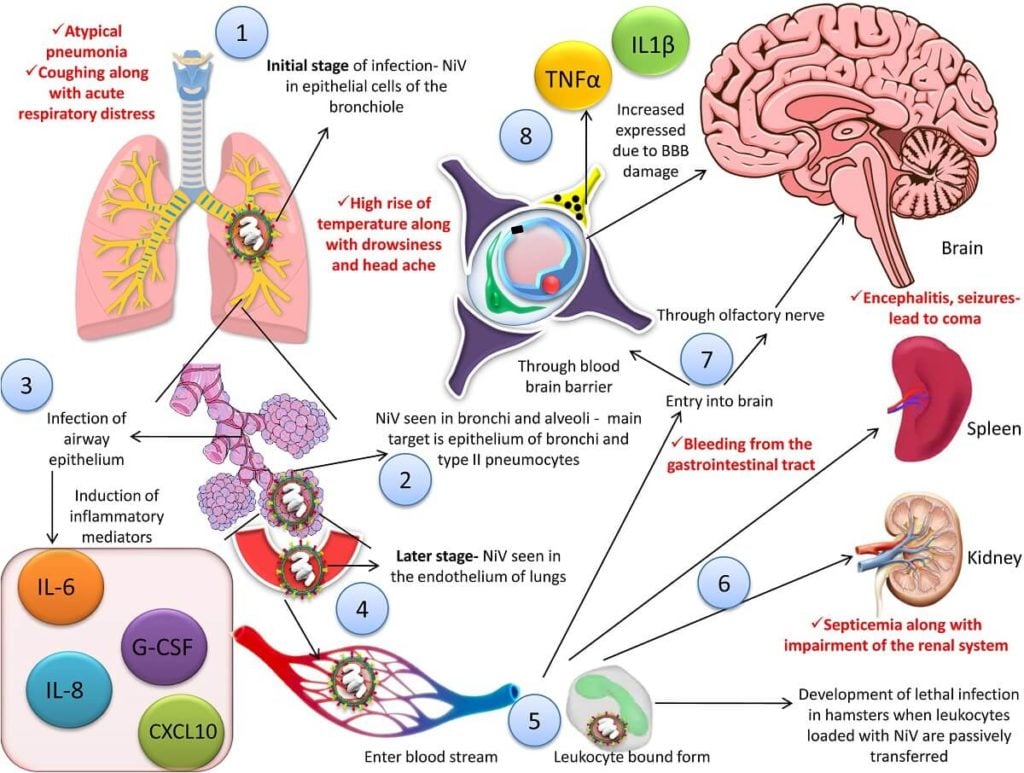
- The NiV enters the host through the oro-nasal route and causes infection.
- The exact site for initial replication in humans is still not clearly understood.
- However, the lymphoid and respiratory tissues are considered the potential initial replication sites due to the high concentrations of antigen found in them during the infection.
- The viral antigens that can be detected in the bronchi and alveoli induce the production of inflammatory cytokines that can ultimately lead to acute respiratory distress syndrome (ARDS)-like disease.
- The virus then gets disseminated to the endothelial cells of the lungs in the later stage of the disease.
- The early viremia leads to the spread of the virus followed by the secondary replication in the endothelium.
- The viral glycoprotein G bind to the receptor Ephrin-B2 on the endothelium as well as lungs, placenta, prostate, blood vessels, and various other tissues.
- Ephrin-B2 is highly conserved between different classes of animals with similarities of approximately 95-96% in bats and pigs which explains the wide range of hosts associated with the Nipah virus.
- The virus invades the central nervous system via the hematogenous circulation and/or anterogradely via olfactory nerves
- It disrupts the blood-brain barrier (BBB) and expresses the IL-1β along with tumor necrosis factor (TNF)-α that results in neurological manifestations during the infection.
- The virus evades the innate immune response resulting in high lethality.
- The P gene products also inhibit interferon activity.
- The different strains of the NiV virus are considered to have coevolved separately with their reservoirs which explains the differences observed in its pathogenicity and epidemiological features.
Clinical Manifestations of Nipah Virus (NiV)
Nipah virus infection can cause mild to severe manifestations, including encephalitis and ultimately death.
The incubation period of the virus generally ranges from 4 to 14 days. However, an extended incubation period of 45 days has also been reported. The general symptoms during the initial phase of the infection include:
- Fever
- Headache
- Cough
- Sore throat
- Difficulty in breathing
- Myalgia
- Vomiting
Severe symptoms may follow which can progressively lead to coma within 24-48 hours. The symptoms may include:
- Disorientation
- Drowsiness
- Confusion
- Seizures
- Coma
- Brain swelling (encephalitis)
Certain patients show brainstem dysfunction like abnormal doll’s eye reflex, pupillary reflexes, vasomotor changes, seizures, and myoclonic jerks.
A characteristic feature of the NiV infection includes relapse or late-onset encephalitis, some of which can occur months or years after the acute infection.
Some patients also develop psychiatric manifestations including depression, personality changes, lack of concentration, and loss of verbal and/or visual memory.
Differences in clinical manifestations were also observed between the different strains during the outbreaks in Malaysia and India.
Respiratory illness like cough, respiratory distress, and atypical pneumonia was observed in 70% of the patients along with a higher mortality rate of 70% in the outbreak in India and Bangladesh as compared to the outbreak in Malaysia where no significant respiratory involvement was observed and had a relatively lower mortality rate of 40%.
Diagnosis of Nipah Virus (NiV)
The specimen for the serological testing should be collected 10-14 days after the onset which may include throat swabs, urine, blood, and/or CSF for diagnosis.
The specimen should be processed in a BSL-4 laboratory.
However, it can also be processed in a BSL-2 laboratory after virus inactivation through sample irradiation.
PCR
The NiV RNA can be detected through Real-Time PCR (RT-PCR) from the respiratory secretions, urine, or CSF. This technique has high sensitivity and specificity. The TaqMan probe-based assay detects the N gene and has a higher sensitivity whereas the SYBR Green-based assay detects a different region of the N gene but has a lower sensitivity and also detects the HeV.
Immunohistochemistry
A wide range of formalin-fixed tissues from different sites including brain, lung, spleen, kidney, and lymph nodes can be used for immunohistochemistry. Convalescent human serum was previously used for immunohistochemistry which has now been replaced by rabbit serum against NiV.
Virus Isolation
The virus isolation from the specimen must be done in a BSL-4 laboratory and the cell line of choice is the Vero cell line. Cytopathic effects are observed within 3 days with the formation of syncytia by NiV that is larger than that formed by the HeV which helps in the differentiation between the two.
ELISA
It is the most common serological method for the detection of NiV infection. It is used to detect IgG and IgM. The IgM antibodies are detectable in 50% of patients on the first day of illness, while 100% of patients show IgG positivity after 18 days. IgG persists for several months.
Serum Neutralization Test
It must be performed in the BSL-4 laboratory but is considered the gold standard test for detection of NiV infection. The Vero cells are infected with the test sera incubated with the virus and observed for cytopathic effects at 3 days.
Treatment of NiV
- The treatment of the NiV infection is limited to supportive treatment and management of acute encephalitis.
- The supportive treatment can include the administration of anticonvulsants, treatment of secondary infection, mechanical ventilation, and rehabilitation.
- Three pharmacological agents have been explored for the possible treatment of NiV which include Ribavirin, m102.4 monoclonal antibodies, and Favipiravir.
Prevention and Control of NiV
Due to the limited treatments available for the NiV infection, the prevention of the disease must be of high priority.
Despite being one of the priority pathogens of the WHO with high fatality rates, there are still no vaccines that are effective and licensed for the NiV infection for human use. However, vaccines against the Nipah virus developed by Public Health Vaccines, the University of Tokyo, and the University of Oxford are in their preclinical trials with plans to test their safety and efficacies in the next few years.
Some preventive measures for the disease include:
- Interventions to prevent farm animals to be infected with the virus by preventing them from eating fruits contaminated with the virus through the fruit bats
- Farms should not be near fruit trees that attract the bats
- Consumption of contaminated sap must be avoided
- Installation of physical barriers to prevent the access of the trees by the bats
- The specimens must be handled in proper biosafety cabinets with minimal aerosol formation procedures
- Patients or farm animals suspected of NiV infection must be immediately isolated
- In high-risk areas for the disease, hospitals, and health care centers must be prepared for the proper screening and management of possible outbreaks
- Proper handwashing and hygiene practice must be exercised before and after handling specimens or contact with the patient
References
- Raj Kumar Singh et al. (2019). Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies – a comprehensive review, Veterinary Quarterly, 39:1, 26-55.
- Ang, B. S. P., Lim, T. C. C., & Wang, L. (2018). Nipah Virus Infection. Journal of Clinical Microbiology, 56(6), e01875-17. https://doi.org/10.1128/jcm.01875-17
- Banerjee, S., Gupta, N., Kodan, P., Mittal, A., Ray, Y., Nischal, N., Soneja, M., Biswas, A., & Wig, N. (2019). Nipah virus disease: A rare and intractable disease. Intractable & Rare Diseases Research, 8(1), 1–8. https://doi.org/10.5582/irdr.2018.01130
- Aditi, Shariff M (2019). Nipah virus infection: A review. Epidemiology and Infection 147, e95, 1–6. https://doi.org/10.1017/S0950268819000086
- Nipah Virus Medical Briefing – Health Reports. (n.d.). Healix. Retrieved March 24, 2022, from https://healix.com/reports/nipah-virus-medical-briefing/
- Nipah Virus (NiV) | CDC. (2019, February 25). Www.cdc.gov. https://www.cdc.gov/vhf/nipah/index.html#:~:text=Nipah%20virus%20(NiV)%20is%20a
- World Health Organization. (2018, May 30). Nipah virus. Who.int; World Health Organization: WHO. https://www.who.int/news-room/fact-sheets/detail/nipah-virus
- Alexandra Benisek. (n.d.). Nipah Virus: What You Should Know. WebMD. https://www.webmd.com/a-to-z-guides/nipah-virus-what-to-know
- Sun, B., Jia, L., Liang, B., Chen, Q., & Liu, D. (2018). Phylogeography, Transmission, and Viral Proteins of Nipah Virus. Virologica Sinica, 33(5), 385–393. https://doi.org/10.1007/s12250-018-0050-1
- Chua KB, Bellini WJ, Rota PA, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science 2000; 288:1432-5.
- Hsu VP, Hossain MJ, Parashar UD, et al. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis 2004; 10:2082-7.
- Wong KT, Shieh WJ, Kumar S, et al. Pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol 2002; 161:2153-67.

The Nipah virus is a deadly virus that has caused multiple outbreaks in Southeast Asia. It’s a zoonotic virus that is transmitted to humans through contact with infected animals, specifically fruit bats. The symptoms of NiV include fever, headache, and respiratory problems, and it can lead to encephalitis, which is inflammation of the brain. There is no specific treatment for NiV, but early intervention can help to mitigate the spread of the virus. It’s important to stay informed and take preventive measures to protect yourself and your loved ones.