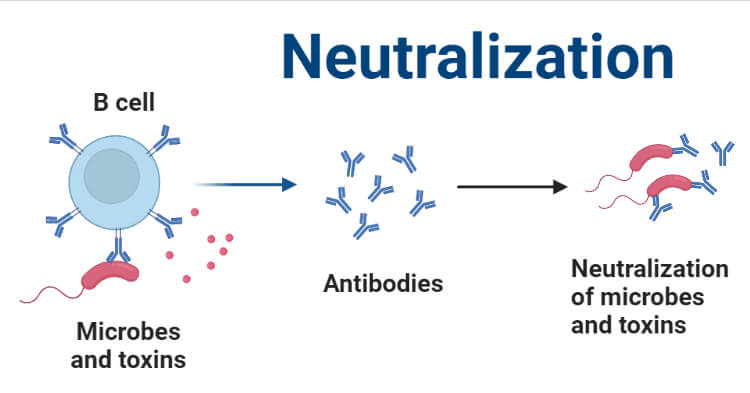
Neutralization means the reduction of the effect of any component. Neutralization is one of the Ag-Ab reactions which aids in reducing the effects of viral antigens or viruses and toxins produced by different disease-causing microbes such as bacterial exotoxins.
There are some specific antibodies involved in neutralization and these antibodies are called neutralizing antibodies. Not all antibodies produced in our body can block the effects of antigens. Only neutralizing antibodies can block the uptake of viral particles or antigens by the cells and neutralize their activity. It belongs to both in-vitro and in-vivo types of Ag-Ab reactions.
Interesting Science Videos
Neutralization Principle
It is based on the principle that some specific antitoxins or antibodies can reduce or neutralize various biological effects occurring due to different enzymes, toxins, and viruses.
Neutralization Types
It is of two types:
Virus Neutralization Test
Virus Neutralization Test is a type of neutralization test used for virus detection. As the name suggests, it is used for the neutralization of the biological activities of the virus.
Viruses can be grown by egg inoculation, animal inoculation, and cell culture. If the antibodies specific for the respective virus are inoculated in these mediums, it can halt the growth and replication of viruses. It is the fundamental principle of the virus neutralization test.
The virus contains certain antigenic determinants to which the antibodies initiate the neutralization of the effects of the virus.
Two examples of Virus Neutralization Test are:
A. Neutralization of Cytopathic Effect
Cytopathic Effect(CPE) is the change that occurs in the structure of the cells of the host invaded by the virus. It can result in the death of the cells. Eg. Poliovirus produces cytopathic effects.
Requirements
Serum sample, Known viral suspension, Cell culture suitable for the virus, Diluting solution
Procedure
The general procedure is as follows:
- The patient’s serum is taken and diluted.
- It is then mixed with the known viral suspension in equal volume and incubated.
- Then the mixture is inoculated in cell culture.
- Incubated and observed for cytopathic effects.
- A control is also prepared to compare the results.
Result Interpretation
If the sample contains neutralizing antibodies then the viral components will be neutralized by it and cytopathic effects won’t be observed after the test. It will be considered a positive test. The occurrence of cytopathic effects will suggest a negative result.
B. Haemagglutination Inhibition Test
Haemagglutination is a process of aggregation or clumping of erythrocytes by the virus. This test relies on the prevention of clump formation. It is a very convenient way of measuring the number of specific antibodies against the virus in the serum sample.
Requirements of Haemagglutination Inhibition Test
Serum sample, Known virus suspension, RBC suspension from a specific source (Eg. For Rubella Virus, RBCs from newborn chickens can be taken. Rubella virus can agglutinate RBCs of newborn chickens), Diluting solution such as saline
Procedure of Haemagglutination Inhibition Test
The general procedure is as follows:
- A serum sample is taken from the suspected patient.
- Serial dilution of the sample is performed in saline solutions.
- Then a typical amount of virus is added to the solution and incubated.
- Then RBC suspension is added to it.
- The components are allowed to mix.
- And finally, the presence or absence of haemagglutination is observed.
Result Interpretation of Haemagglutination Inhibition Test
If the patient’s serum contains neutralizing antibodies then haemagglutination won’t be observed in the sample and it will be considered a positive test.
The neutralizing antibodies bind to different binding sites on the virus and won’t let it bind with RBC preventing the haemagglutination.
The extent of haemagglutination in different dilutions will help to determine the severity of infection or the amount of neutralizing antibodies.
Applications of Virus Neutralization Test
- Covid-19 neutralizing antibodies test is used to detect the respective infection in an individual and its severity.
- It can be used to detect various viral infections such as measles, mumps, influenza, etc.
Toxin Neutralization Test
The toxins produced by microbes are harmful as they can alter different biological functions of our body once injected within. But there are certain antitoxins prepared specifically to reduce the biological effects of those toxins. Utilization of these antitoxins for specific toxin neutralization is the basis of this test.
Applications of Toxin Neutralization Test
- In vivo use:
- One of the tests named the Schick test is used commonly for the demonstration of immunity to diphtheria infection.
- Another common test is for the neutralization of toxins produced by Clostridium welchii.
- In vitro use:
- It is used for the rapid detection of Clostridium spp by Nagler’s reaction.
Note: Nagler’s reaction or test is used for the detection of alpha-toxin or lecithinase enzyme-producing Clostridium spp. Lecithinase can digest animal tissues.
- Anti Streptolysin O test is also one of the tests based on toxin neutralization.
Procedure of Nagler’s reaction
The general procedure is as follows:
- Egg yolk agar plate is prepared.
- A line is drawn dividing the plate into two halves.
- Antitoxin is spread on one-half of the agar plate.
- Then the test organism is streaked on the whole agar plate.
- It is then incubated in an anaerobic incubator at 37 °C for one or two days.
- The result is interpreted after incubation.
Requirements
Egg Yolk Agar, Known Antitoxin, Inoculum of Test Organism, Anareboic jar or incubator.
Note: The egg contains a component called lecithin which can be dissolved by lecithinase(alpha-toxin) produced by bacteria such as C. perfringens.
Result Interpretation
If the opaque zone is observed in the antitoxin-free area and no opaque zone is observed in the antitoxin inoculated area, the test will be considered positive and vice-versa for the negative test.
Note: The activity of alpha-toxin (lecithinase) is neutralized by antitoxin so the respective area on the agar won’t show an opaque zone. But the activity of toxin occurs in the antitoxin-free area hence lecithinase degrades the lecithin of egg yolk agar and an opaque zone is seen.
Applications of Neutralization Test
- It is used for the determination of pathogenic components such as toxins and viruses and the differentiation between their pathogenicity.
- It can be used in the study of antigenic relations of different viruses and toxins.
- It can also be used to estimate the ability of different vaccines to produce immunity in our bodies and the effectiveness of drugs.
- It can be used to determine the ability of antitoxins to neutralize any toxins produced by pathogenic microbes.
Advantages of Neutralization Test
- It has higher sensitivity.
- It has higher specificity.
- The neutralizing antibodies used in virus neutralization tests can detect the virus along with its various strains.
Limitations of Neutralization Test
- The neutralizing antibodies take time to generate in the body after an infection.
- These tests require professionally skilled individuals to perform.
- It requires a great deal of work.
- The materials required for it can be hard to produce and manage.
- These tests require highly protective lab conditions and care as working with the virus can be hazardous.
References
- Hitchner S. B. (1973). A virus neutralization screening test: its limitations in classifying field isolates of infectious bronchitis virus. Avian pathology: journal of the W.V.P.A, 2(2), 103–109. https://doi.org/10.1080/03079457309353788
- Thullier P., Sesardic D.T. (2010) Neutralization Tests. In: Kontermann R., Dübel S. (eds) Antibody Engineering. Springer Protocols Handbooks. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-01144-3_45.
- Parija S.C., (2009), Textbook of Microbiology and Immunology, 2nd edition, Elsevier, a division of Reed Elsevier India Private Limited.
- Melissa Ann Bourgeois, J. Lindsay Oaks, Equine Infectious Diseases (Second Edition), 2014.

Very well written and all round note. Thank you.