Mycolic acid is the cell wall that contains α-alkylated β-hydroxylated fatty acids present in Mycobacteria, Nocardia, and Corynebacteria. Mycolic acids are the main component of the cell wall of Mycobacterium spp and plays important role in the virulence and permeability of the outer membrane. To control the cell fluidity, trans-cyclopropanes are present in Mtb in mycolate series but in virulent series, oxygenated forms are present along with trans-cyclopropane. Disrupting the mycolic acid pathway with the help of effective treatment is a great task and proven therapy by the drugs like isoniazid, ethionamide, isoxyl, thiacteazone, and triclosan.
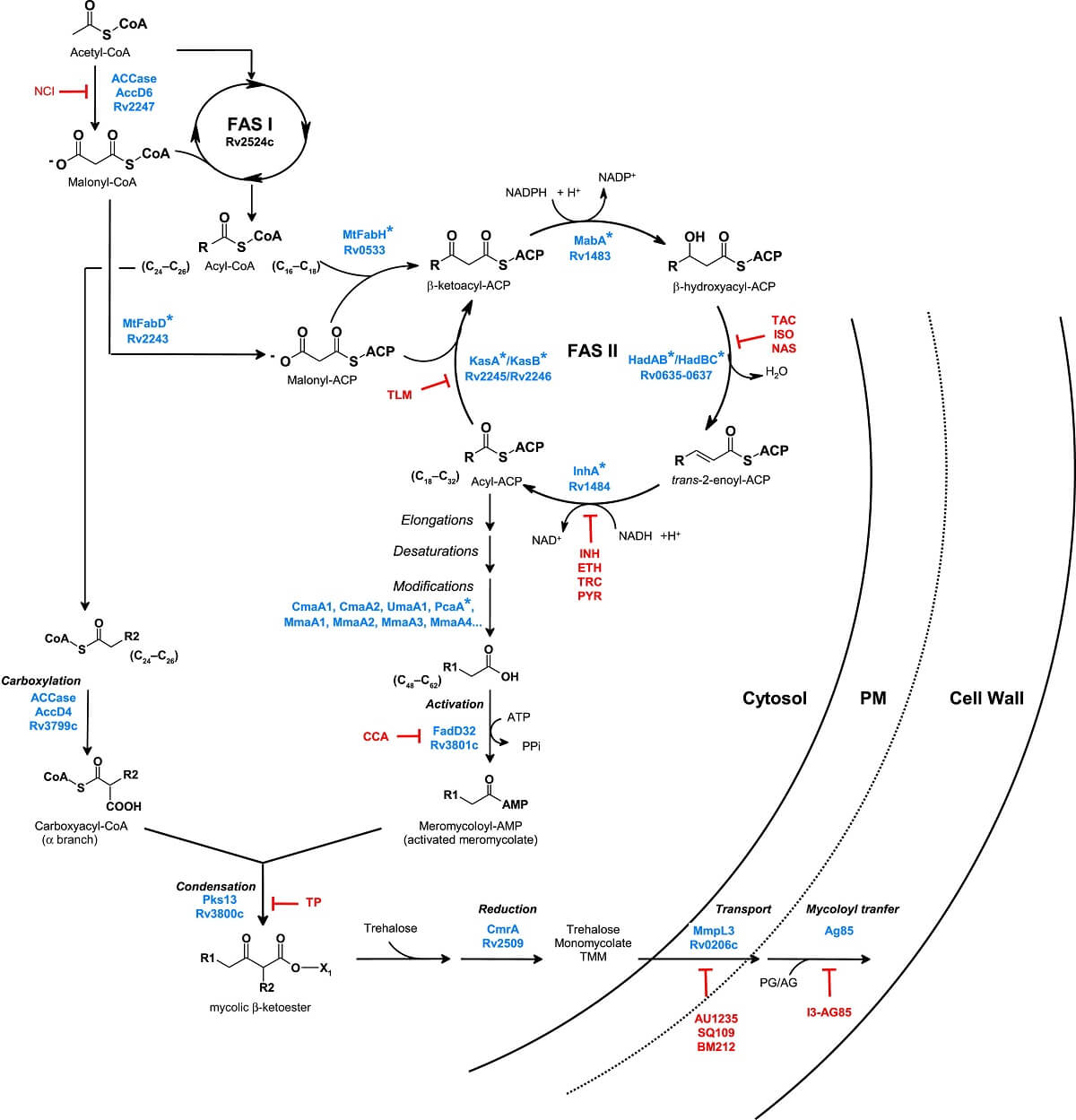
Figure: Mycolic acid Biosynthesis, Inhibition, and Regulation. Image Source: Hedia Marrakchi et al. 2014.
Isoniazid: It is mainly used to treat infections caused by Mycobacterium spp. and used with other antimicrobials to treat infections. It is manufactured from isonicotinic acid which was produced from methylpyridine. It is active against both extracellular and intracellular organisms which penetrate macrophages and inhibit organisms for multiplication. Its structure is similar to pyridoxine.
Ethionamide: It is the second-line anti-TB drug, an analog of isoniazid. It was isolated from Streptomyces orchidaceous. It is lipid-soluble and its activity is enhanced by the ethyl group. It is used in case of resistance of the first-line of anti-tubercular drugs. It is used in combination with isoniazid or streptomycin after the resistance showed against Mycobacterium tuberculosis.
Isoxyl: Isoxyl or thiocarlide was used to treat tuberculosis in the 1960s. It inhibits the mycolic acid synthesis pathway in Mycobacterium bovis and Mycobacterium tuberculosis.
Thioacetazone: An antibiotic that is used for the treatment of pulmonary tuberculosis. After the usage and discovery, its usage is reduced because of the toxicity seen in patients.
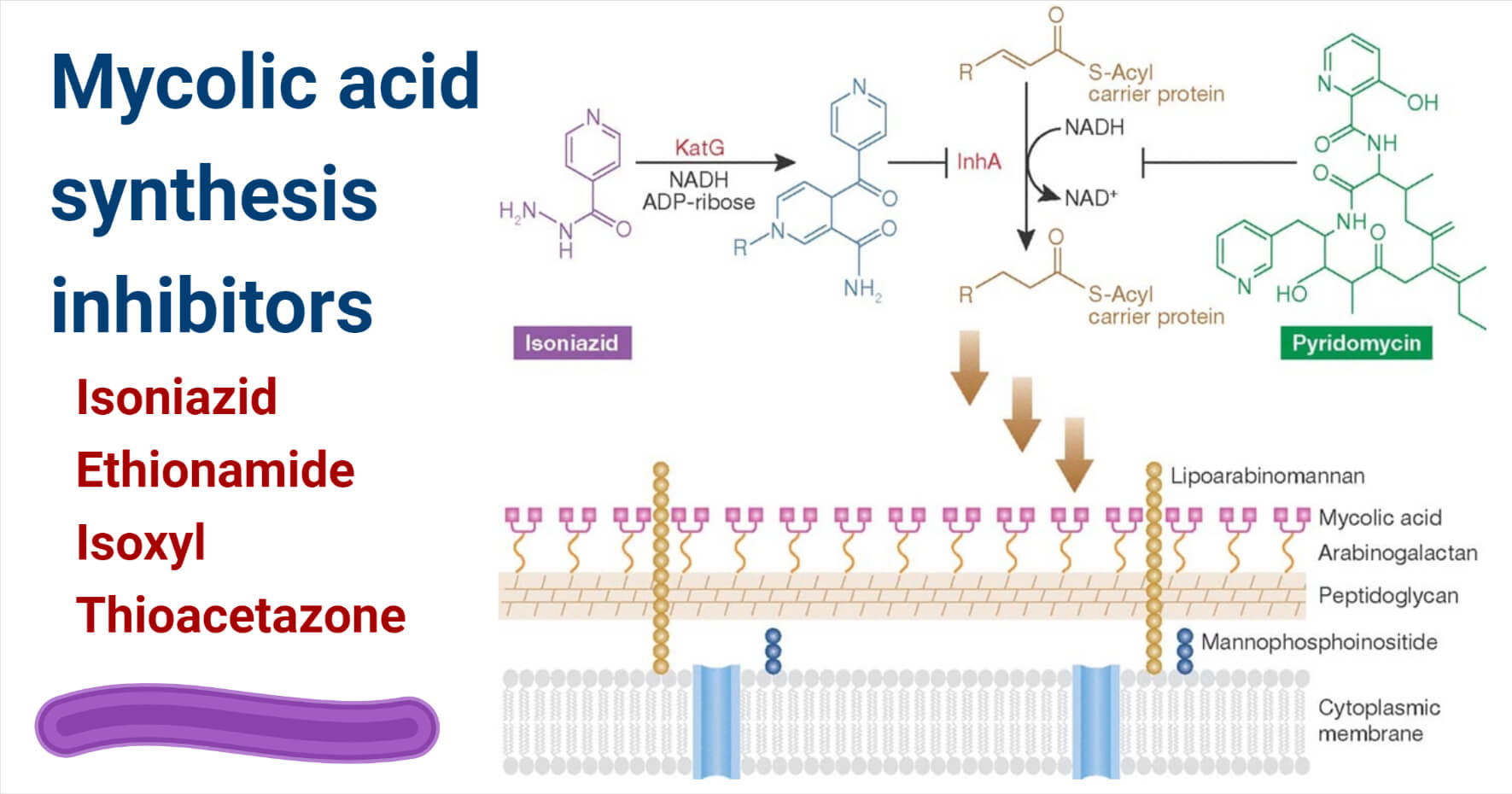
Figure: Inhibition of mycolic acid synthesis by isoniazid and pyridomycin. Image Source: Gerard D. Wright 2012.
Interesting Science Videos
Mechanism of action of Mycolic acid biosynthesis inhibitors
Isoniazid: Isoniazid is the first-line drug used to treat a mycobacterial infection that targets enoyl-ACP reductase. Catalase KatG is activated by isoniazid to a free radical form that binds to NAD forming an isonicotinyl NAD complex This formed complex is a reductase inhibitor, InhA.
Ethionamide: It acts similar to isoniazid is a nicotinic acid derivative that undergoes intracellular alteration. It requires oxidative activation done by EthA before inhibition done by InhA. Ethionamide reacts with NAD forming and ethionamide NAD complex that inhibits InhA. EthA is a FAD containing monooxygenase. Catalase peroxidase is converted into ethionamide sulfoxide upon oxidization with ethionamide. It further inactivates enoyl reductase and acylates cystine in InhA protein.
Isoxyl: The main effect shown by isoxyl is inhibition of synthesis of tuberculostearic and oleic acid which is directly related to the effect shown by a drug. The formation of 3-hydroxy C18, C20, and C22 fatty acids shows the inhibition of the dehydratase step in the fatty acid synthase II elongation cycle.
Thioacetazone: It is a weak antitubercular drug and it inhibits bacteria by mycolic acid cyclopropane synthesis. It inhibits cyclopropanaton of the cell walls which shows its bacteriostatic nature.
Examples of Mycolic acid biosynthesis inhibitors
- Isoniazid, Ethionamide, etc are some mycolic acid synthesis inhibitors.
Mechanism of Resistance of Mycolic acid biosynthesis inhibitors
Mainly drug resistance occurs due to the efflux pumps and permeability barrier but here are some other reasons included;
Isoniazid
- Mutations in katG, inhA, and most frequently in the promoter region cause resistance to isoniazid.
- Loss of catalase/ peroxidase activity is the most common genetic alteration that results in resistivity.
- Downregulation of katG has also been reported.
- Substitution of serine at 315 results in isoniazid resistance in many clinical isolates.
Ethionamide
- Mutations in inhA and ethA show resistance in ethionamide. Mutation targets inhA target or the overexpression of the gene.
References
- Dick T and Shetty A (2018). i v o r l a n o l. https://doi.org/10.3389/fmicb.2018.01898
- Einstein A. (2019). HHS Public Access. 2(4). https://doi.org/10.1128/microbiolspec.MGM2-0014-2013.Resistance
- From, Isoniazid: Uses, Interactions, Mechanism of Action | DrugBank Online
- From, Ethionamide: Uses, Interactions, Mechanism of Action | DrugBank Online
- Kordula J, Janin Y L, Liav A, Barilone N, Vultos T. Dos, Rauzier, J, Jackson, M. (2007). Isoxyl Activation Is Required for Bacteriostatic Activity against Mycobacterium tuberculosis 51(11): 3824–3829. https://doi.org/10.1128/AAC.00433-07
- North E J, Jackson, M and Lee R E. (2015). HHS Public Access. 20(27):4357–4378.
- Palomino J C and Martin, A. (2014). Drug Resistance Mechanisms in Mycobacterium tuberculosis. 317–340. https://doi.org/10.3390/antibiotics3030317
- Phetsuksiri B, Baulard A, Cooper A M, Minnikin D E, Douglas JD, Besra G S and Brennan, P J. (1999). Antimycobacterial Activities of Isoxyl and New Derivatives through the Inhibition of Mycolic Acid Synthesis. 43(5): 1042–1051.
- Takayama K and Davidson LA. (1979). Antimycobacterial drugs that inhibit mycolic acid synthesis. (1972), 280–282.
- Timmins G S and Deretic V. (2006). MicroReview Mechanisms of action of isoniazid. 62(October): 1220–1227. https://doi.org/10.1111/j.1365-2958.2006.05467.xUnissa, A. N., Subbian, S, Elizabeth L and Selvakumar, N. (2016). Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. MEEGID. https://doi.org/10.1016/j.meegid.2016.09.004
- Wang F, Langley R., Gulten G, Dover L G, Besra GS., Jr, W. R. J and Sacchettini J C. (2007). Mechanism of thioamide drug action against tuberculosis and leprosy. 204(1), 73–78. https://doi.org/10.1084/jem.20062100.
- Marrakchi H, Lanéelle MA, Daffé M. Mycolic acids: structures, biosynthesis, and beyond. Chem Biol. 2014 Jan 16;21(1):67-85. doi: 10.1016/j.chembiol.2013.11.011. Epub 2013 Dec 26. PMID: 24374164.
- Wright GD. Back to the future: a new ‘old’ lead for tuberculosis. EMBO Mol Med. 2012 Oct;4(10):1029-31. doi: 10.1002/emmm.201201811. Epub 2012 Sep 17. PMID: 22987753; PMCID: PMC3491833.
