Interesting Science Videos
Structure of Mumps Virus
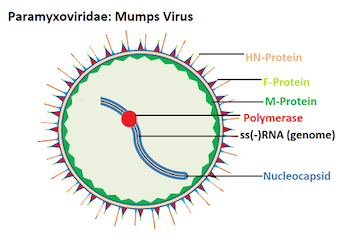
- Mumps virions are markedly pleomorphic (100 to 600 nm), and roughly spherical with an outer membrane that envelops an inner, coiled helical structure.
- The envelope which is 150 to 300nm is studded with projections formed by the viral glycoproteins that rise 12 to 15 nm from the virion surface.
- The coiled inner structure or nucleocapsid is a ribonucleoprotein complex that appears to be a hollow tube with a unit length of approximately 1 μm, a diameter of 17 nm, and a central core of 5 nm.
- The nucleocapsid consists of the negative-sense, single-stranded RNA associated with the nucleoprotein (NP), polymerase phosphoprotein (P), and large (L)
- The L protein is the RNA polymerase, the P protein facilitates RNA synthesis, and the NP protein helps maintain genomic structure.
- The nucleocapsid associates with the matrix (M) protein lining the inside of the virion envelope.
- The envelope contains two glycoproteins, a fusion (F) protein, which promotes fusion of the viral and host cell membranes, and a viral attachment protein (hemagglutinin-neuraminidase [HN], hemagglutinin [H], or glycoprotein [G] protein).
- Full-length genomic RNA is an unsegmented, single-stranded macromolecule of negative polarity.
Genome of Mumps Virus
- The genome is monopartite, unsegmented single stranded RNA with negative polarity that contains about 15.3 kilobases.
- The virus contains six major structural proteins: nucleocapsid-associated protein (NP), phosphoprotein (P), and polymerase protein (L) are associated with the nucleocapsid; membrane or matrix (M) protein; and two glycoproteins, a hemagglutinin-neuraminidase (HN) and a fusion (F) molecule.
- The virus genes have been sequenced and the gene order for mumps virus is 3’-NP–P–M–F–SH–HN–L-5’.
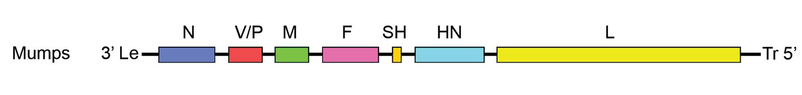
- There is a nontranscribed leader sequence of 55 nucleotides and a trailing sequence of 24 nucleotides that share inverse complementarity at their extremes.
Epidemiology of Mumps Virus
- Mumps occurs endemically worldwide.
- Cases appear throughout the year in hot climates and peak in the winter and spring in temperate climates.
- Mumps is primarily an infection of children, with the highest incidence in children ages 5–9 years.
- Outbreaks occur where crowding favors dissemination of the virus.
- In children younger than 5 years, mumps may commonly cause upper respiratory tract infection without parotitis.
- The overall mortality rate for mumps is low (one death per 10,000 cases in the United States), mostly caused by encephalitis.
- The incidence of mumps and associated complications has declined markedly since introduction of the live-virus vaccine.
- In 1967, the year mumps vaccine was licensed, there were about 200,000 mumps cases (and 900 patients with encephalitis) in the United States.
- From 2001 to 2003, there were fewer than 300 mumps cases each year.
- In 2006, there was an outbreak of mumps in the United States that resulted in more than 5700 cases.
- In 2009, a mumps outbreak occurred in the states of New York and New Jersey in which 88% of those affected had been vaccinated.
- A massive mumps epidemic occurred in 2004 in the United Kingdom that caused more than 56,000 cases.
Replication of Mumps Virus
- Virions attach via the HN protein to cellular sialoglycoproteins or glycolipid receptors.
- The F protein then mediates fusion of the viral envelope with the plasma membranes at physiological pH and ribonucleocapsid is released in the cytoplasm.
- The liberated nucleocapsid remains intact, and all three associated proteins (N, P, and L) are required for the transcription of the incoming viral genome.
- This nucleocapsid structure thus acts as a template for the initiation of infection through a process of transcription of viral mRNA from the negative-sense viral RNA.
- During the early stages of the replication cycle, the viral genome directs the synthesis of a (+) sense leader and six to ten discrete, unprocessed mRNA species.
- The mRNAs are capped and polyadenylated.
- When the concentration of N protein reaches a critical level it binds to the nascent (+) sense antigenome intermediates, with the result that the polyadenylation, termination, and reinitiation of full-length RNA molecules is prevented and transcription then switches to the replication of full-length genome intermediates: these in turn serve as templates for the production of nascent full-length (–) sense viral genomes.
- Newly synthesized RNA associates with the N proteins and L protein to form helical nucleocapsids.
- The ribonucleocapsid interacts with the matrix protein under the plasma membrane and buds via the ESCRT complex, releasing the virion.
Pathogenesis of Mumps Virus
- Natural infection is initiated by droplet spread.
- Primary replication occurs in nasal or upper respiratory tract epithelial cells.
- Viremia then disseminates the virus to the salivary glands and other major organ systems.
- The incubation period may range from 2 to 4 weeks but is typically about 14–18 days.
- Virus is shed in saliva for as long as 6 days before the onset of parotitis.
- About one-third of infected individuals does not exhibit obvious symptoms but are equally capable of transmitting infection.
- Termination of viral excretion in saliva correlates with the local appearance of virus-specific secretory IgA, as early as 5 days after disease onset.
- Virus-specific IgM antibodies are also present early in saliva.
- Thus, patients with mumps are capable of spreading virus by the respiratory route over a 7 to 10 days interval.
- A cell-mediated immune response also develops.
- Interferon is induced early in mumps infection.
- Plasma viremia disappears coincident with the development of mumps virus–specific humoral antibody, which can be detected in serum as early as 11 days after induced apparent or inapparent infection of humans.
- Mumps virus preferentially infects activated human T lymphocytes.
- Mumps is a systemic viral disease with a propensity to replicate in epithelial cells in various visceral organs.
- Virus frequently infects the kidneys and can be detected in the urine of most patients.
- Viruria may persist for up to 14 days after the onset of clinical symptoms.
- The central nervous system is also commonly infected and may be involved in the absence of parotitis.
- Viral invasion of the CNS presumably occurs across the choroid plexus.
- Blood-borne infected mononuclear cells may cross the fenestrated endothelium of the choroid plexus stroma and serve as a source for subsequent infection of the choroidal epithelium.
- Maturation of virus from the ventricular surfaces of choroidal cells provides progeny virions that are widely distributed through ventricular pathways and the subarachnoid space by CSF.
Clinical manifestations of Mumps Virus
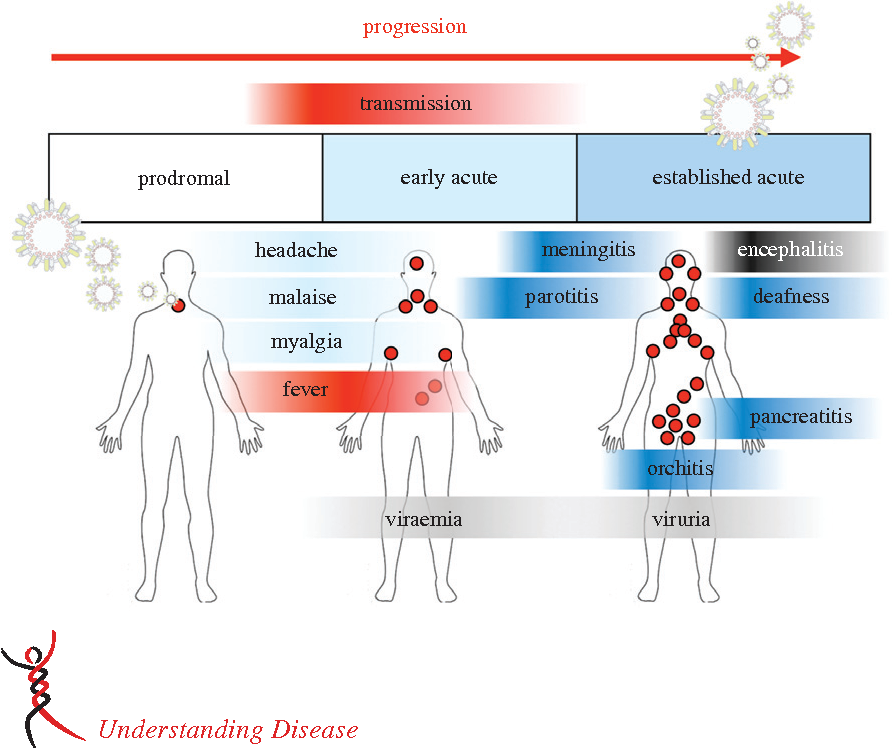
- At least one-third of all mumps infections are subclinical, including the majority of infections in children younger than 2 years.
- The most characteristic feature of symptomatic cases is swelling of the salivary glands, which occurs in about 50% of patients.
- Virus infects the ductal epithelium, producing intracytoplasmic inclusions (viral nucleocapsids) and viral antigens, with eventual desquamation of involved cells.
- A prodromal period of malaise and anorexia is followed by rapid enlargement of parotid glands as well as other salivary glands.
- Swelling, inflammation, and tissue damage in the parotid gland may produce elevation of serum and urine amylase levels.
- Virus also frequently disseminates to the kidneys, where epithelial cells of the distal tubules, calyces, and ureters appear to be primary sites of virus replication.
- Viruria, as an expression of renal involvement, can be detected in most patients, sometimes for as long as 14 days after the onset of clinical symptoms.
- Central nervous system involvement is common (10–30% of cases).
- Mumps causes aseptic meningitis and is more common among males than females.
- Meningoencephalitis usually occurs 5–7 days after inflammation of the salivary glands, but up to half of patients will not have clinical evidence of parotitis.
- Cases of mumps meningitis and meningoencephalitis usually resolve without sequelae, although unilateral deafness occurs in about five in 100,000 cases.
- The testes and ovaries may be affected, especially after puberty.
- Around 20–50% of men who are infected with mumps virus develop orchitis (often unilateral).
- Atrophy of the testis may occur as a result of pressure necrosis but only rarely does sterility result.
- Mumps oophoritis occurs in about 5% of women.
- Pancreatitis is reported in about 4% of cases.
- Pancreatic involvement is usually expressed as mild epigastric pain, but severe hemorrhagic pancreatitis and transient exocrine function abnormalities have also been reported.
- Mumps may produce a fetal wastage when contracted by the mother during the first trimester of pregnancy, occurring after blood-borne infection of the placenta, with or without subsequent spread of virus to involve fetal tissues directly.
- A proliferative necrotizing villitis with decidual cells containing intracytoplasmic inclusions has been described in the products of spontaneous and induced abortions.
Laboratory Diagnosis of Mumps Virus
Specimen
- Virus isolation from saliva is most successful during the first 4 to 5 days of symptoms.
- Virus is recovered from urine for up to 2 weeks after symptoms begin, especially if care is taken to first clear the urine of debris, then concentrate the virus by ultracentrifugation.
- In patients with meningitis, the CSF can be expected to yield virus in, at best, about half of samples taken within 8 to 9 days of the onset of CNS disease.
Virus isolation
- Monkey kidney primary or cell lines may be used for isolation, although other continuous cell lines are nearly comparable in sensitivity.
- Samples should be inoculated shortly after collection because mumps virus is thermolabile.
- For rapid diagnosis, immunofluorescence using mumps-specific antiserum can detect mumps virus antigens as early as 2–3 days after the inoculation of cell cultures in shell vials.
- Not all primary mumps virus isolates show characteristic syncytial cytopathic effects.
- Therefore, hemadsorption techniques should be applied to all cultures before they are discarded as negative.
- Immunofluorescence techniques not only provide direct confirmation of the isolation of a hemagglutinating virus in the culture but also, with appropriate reagents, specify the isolate as mumps virus.
Nucleic Acid Detection
- RT-PCR is a very sensitive method that can detect mumps genome sequences in clinical samples.
- It can detect the virus in many clinical samples that have negative results in virus isolation attempts.
- RT-PCR assays can identify virus strains and provide useful information in epidemiologic studies.
Serology
- Simple detection of mumps antibody is not adequate to diagnose an infection.
- Rather, an antibody rise can be demonstrated using paired sera: a fourfold or greater rise in antibody titer is evidence of mumps infection.
- The ELISA or HI test is commonly used.
- Antibodies against the HN protein are neutralizing.
- ELISA can be designed to detect either mumps-specific IgM antibody or mumps-specific IgG antibody.
- Mumps IgM is uniformly present early in the illness and seldom persists longer than 60 days.
- Therefore, demonstration of mumps specific IgM in serum drawn early in illness strongly suggests recent infection.
Treatment of Mumps Virus
There is no specific therapy for mumps virus.
Prevention and control of Mumps Virus
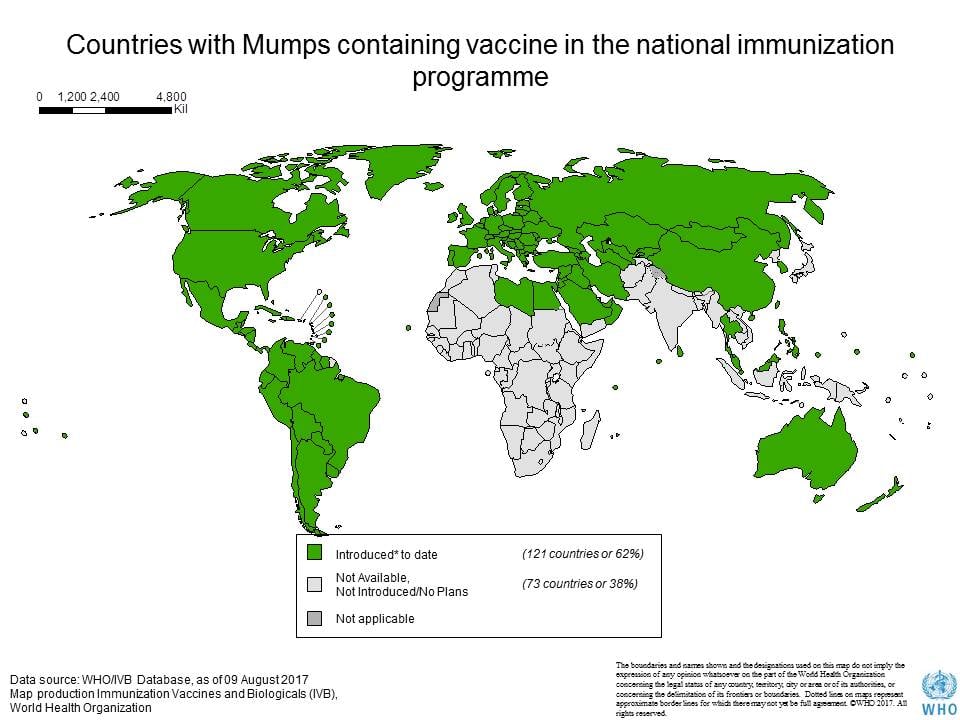
- Prevention through an effective vaccination program is the rational approach to the control of mumps.
- Immunization with attenuated live mumps virus vaccine is the best approach to reducing mumps-associated morbidity and mortality rates.
- The use of live attenuated mumps virus vaccines has caused a remarkable decline in the incidence of mumps and related sequelae of wild-type infection.
- Some, but not very reliable, protection can be given by passive immunization, which may prevent severe orchitis even when given at the stage of parotitis.
- Mumps vaccine, based on the Jeryl Lynn or Urabe strains, has been available as a monovalent vaccine for some time, particularly in the USA, but is now widely incorporated into the triple MMR vaccine.
- All three components are live-attenuated viruses, and the mumps component induces good antibody levels, lasting long enough to suggest that the recipients will not become susceptible as adults.
- An effective attenuated live-virus vaccine made in chick embryo cell culture was licensed in the United States in 1967.
- It produces a subclinical, noncommunicable infection.
- Mumps vaccine is available in combination with measles and rubella (MMR) live-virus vaccines.
- Combination live-virus vaccines produce antibodies to each of the viruses in about 78–95% of vaccinees.
- There is no increased risk of aseptic meningitis after MMR vaccination.
- Two doses of MMR vaccine are recommended for school entry.
- Control measures for mumps must be primarily directed at prevention.