Mucormycosis is an infection caused by several species of filamentous molds belonging to the order Mucorales.
- The infections usually occur in immunocompromised individuals with one or more underlying conditions.
- The fungi responsible for these infections are found in different environmental niches like soil, decaying vegetables, bread, and even dust.
- Some of the risk factors associated with mucormycosis include conditions like uncontrolled diabetes mellitus, bone marrow transplant, neutropenia, trauma, burns, and hematologic disorders.
- Studies related to mucormycosis have increased over the years due to the severity of these infections, with a high rate of mortality.
- Some of the species belonging to the order Mucorales are Rhizopus, Mucor, Rhizomucor, Apophysomyces, etc. Rhizopus is the most common species associated with mucormycosis, closely followed by Mucor and Lichtheimia.
- The infections can be characterized by different clinical manifestations depending on the site of infection and the severity.
- Some of the common sites of infections by Mucorales include sinuses, lungs, skin, and gastrointestinal tract.
- Mucormycosis has also been associated with molds from the order Entomophthorales; however, these infections are not angioinvasive and do not disseminate. Such molds result in chronic subcutaneous infections even in immunocompetent hosts.
Interesting Science Videos
Habitat of order Mucorales
- The fungal species belonging to the order Mucorales can be found throughout the environment in different sources ranging from soil to vegetables.
- Even though these species are ubiquitous in distribution, they are predominantly saprobic soil organisms.
- The fungi can be commonly found in soil than in air as these exist in the form of spores in order to protect themselves as well as to assist the process of dispersal. The occurrence thus is more prevalent in tropical areas.
- The dispersal and occurrence of these species are more common during summer than in winter as the fungal spores thrive in dry and arid conditions.
- Besides, some of these fungi can also occur in decaying matter like decaying vegetables and fruits as these are good sources of carbohydrates that are essential for the growth and survival of the species.
- Mucoralean fungi usually reproduce anamorphically via non-motile sporangiospores released from different sporangia.
- Some of the Mucoralean fungi can also occur as parasites of plants, fungi, and animals, resulting in different forms of diseases.
Etiology of Mucormycosis
- The fungal species that are most frequently isolated from patients with Mucormycosis are Apophysomyces, Cunninghamella, Lichtheimia, Mucor, Rhizopus, and Rhizomucor.
- The etiology of these infections differs considerably in different countries, but Rhizopus spp is the most common cause of these infections in most parts of the world.
- These species exist as spores and thrive in dry, humid, and arid conditions. These transmit through the air and result in mild to severe infections in immunocompromised individuals.
- The species present in the order Mucorales display only a small number of distinguishable morphological characteristics that can be used to distinguish between themselves.
- Most of these species are differentiated based on characteristics like structure, size, and shape of the sporangia, color and state of the spores, and the mycelium.
- The Mucoralean fungi are defined by usually abundant and rapidly growing mycelium and other anamorph structures.
- The mycelium is unsepted or irregularly septed, and the anamorphic sporangiospores produce multi-spored sporangia.
- Structures like chlamydospores, arthrospores, and yeast cells are rare in these species. The sporangia consist of the variously shaped columella.
- Some species might exhibit appendages that enable them to switch between the filamentous multicellular state and the yeast-like state.
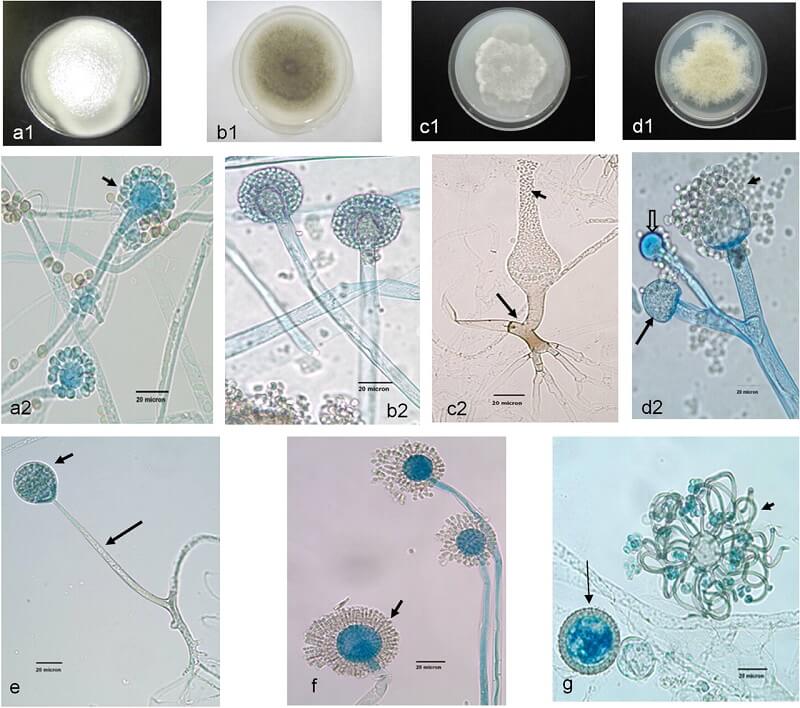
Figure: Unusual Mucormycetes. (a2, b2, c2, d2, and e to g) Lactophenol cotton blue mount preparations. (a1, b1, c1, and d1) Potato dextrose agar (PDA) medium plates. (a1) Cunninghamella bertholletiae colony surface on a PDA medium plate. (a2) C. bertholletiae sporangiophores in terminal swellings called vesicles, with sporangioles (short arrow). (b1) Colony surface of Rhizomucor pusillus on a PDA medium plate incubated at 30°C for 96 h. (b2) R. pusillus sporangiophores with globose sporangia. (c1) Saksenaea vasiformis colony surface on a PDA medium plate incubated at 30°C (48 h). (c2) S. vasiformis sporangiophore arising from a “foot cell”-like hyphal element (long arrow), flask-shaped sporangium, and liberated sporangiospores (short arrow). (d1) Actinomucor elegans colony surface on a PDA medium plate incubated at 30°C (96 h). (d2) Actinomucor elegans branched sporangiophores, sporangium (long arrow), columella (block arrow), and various sporangiospores (short arrow). (e) Unbranched Apophysomyces elegans sporangiophore (long arrow) with a pyriform sporangium (short arrow). (f) Syncephalastrum racemosum sporangiophores with merosporangia (short arrow). (g) Cokeromyces recurvatus sporangiolating vesicle (short arrow) and zygospores (long arrow). Bars, 20 μm. Image Source: Clinical Microbiology Reviews®, American Society for Microbiology.
Virulence Factors of Mucorales
There is a difference in virulence across different species belonging to the order Mucorales, which indicates an array of virulence factors, resulting in aggressive invasive disease in some species and infrequent mortality in others. The following are some of the virulence factors employed by the fungal species responsible for mucormycosis;
Iron overload
- Mucoralean fungi flourish in iron-rich environments as iron is required for cell growth and development as well for different vital processes in the cell.
- It has been observed that the increased level of iron in the serum plays an important role in predisposing patients to mucormycosis.
- Fungi take up the iron from the blood by using iron permeases or chelators and reduce them from ferric to the more soluble ferrous form.
- The ferrous iron generated from the permeases is then captured by a protein complex made up of multicopper oxidase and a ferrous permease.
- The iron take-up by the fungi is essential for enhancing the growth and development of the fungi and increasing their pathogenicity.
High-affinity iron permease (FTR1)
- High-affinity iron permease plays an essential role in iron uptake and transfers within the fungal species, especially in environments with a lack of iron.
- The FTR1 gene is highly expressed in the species during infection by Rhizopus oryzae, and the knockdown of the gene is known to reduce the virulence of the species.
- The permeases occur in fungi as a part of a reductive system containing redundant surface reductases involved in the reduction of ferric to the soluble ferrous form.
Rhizoferrin
- Rhizoferrin is a siderophore secreted by Rhizopus as a part of the polycarboxylate family. The siderophore is responsible for the supply of iron through a receptor-mediated, energy-dependent process.
- However, siderophore on its own is inefficient in obtaining iron from the serum and requires the involvement of the organisms’ endogenous siderophores for virulence.
- In some Mucoralean fungi, the fungus utilizes xenosiderophores like deferoxamine in order to effectively obtain iron from the host.
Calcineurin
- Calcineurin is calcium and calmodulin-dependent serine/threonine protein phosphate that is an essential virulence factor in the pathogenesis of Mucorales.
- It is involved in the transition of Mucor circinelloides from the yeast form to hyphae. The spores of the species are capable of inhibiting phagosomal maturation by macrophages, unlike the yeast cells.
- Calcineurin is also closely related to protein kinase A activity which is an equally important factor for the pathogenesis of M. circinelloides.
Spore coat protein
- Spore coat protein is also a virulence factor that is found universally on the spore of all Mucorales.
- The protein plays an important role as invasions during the pathogenesis of mucormycosis. It also disrupts and damages immune cells and acts as a specific ligand for the GRP78 receptor.
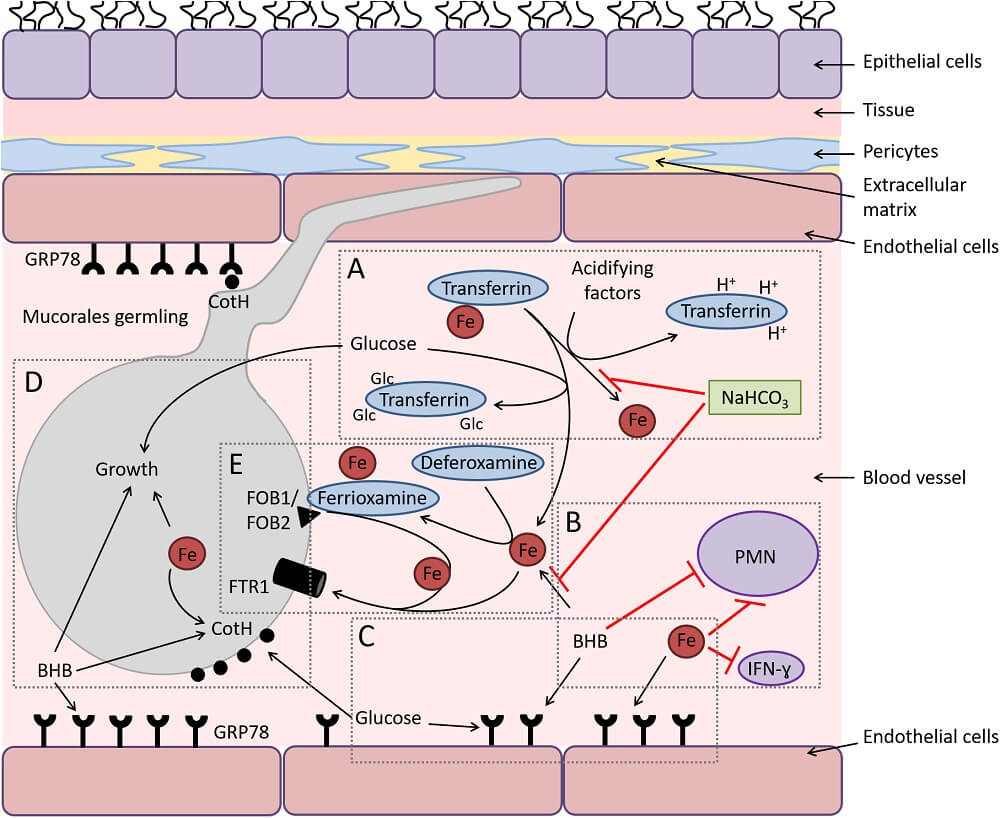
Figure: Diagram depicting the interactions of Mucorales with endothelial cells during hematogenous dissemination/organ seeding and the effect of host factors on these interactions and on the immune response. (A) Hyperglycemia and ketoacidosis result in liberation of iron from serum-sequestering proteins (e.g., transferrin) via glycosylation and protonation, respectively. (B) Ketone bodies (e.g., β-hydroxy butyrate [BHB]) and free iron negatively affect the immune response to the infection, while sodium bicarbonate (NaHCO3) reverses this negative effect by preventing iron release from transferrin and neutralizing acidity. (C) Surface expression of glucose-regulator protein 78 (GRP78) on endothelial cells is enhanced to cope with the stress elicited by hyperglycemia, free iron, and ketone bodies. (D) Glucose, free iron (transported by the high-affinity iron permease [Ftr1p]), and BHB also enhance the expression of fungal cell surface CotH, which results in the invasion of the endothelium and augmentation of fungal growth. (E) In deferoxamine-treated hosts, the iron-rich ferrioxamine binds to its fungal receptor (ferrioxamine binding proteins [Fob1/Fob2]) then releases iron via a reductive step prior to feeding invading Mucorales via Ftr1p transportation. Image Source: PLOS Pathogens.
Mode of Transmission of Mucormycosis
- Mucormycosis is acquired by immunocompromised individuals, mostly by the inhalation of fungal spores from the environment.
- The primary mode of transmission of Mucorales is the inhalation of sporangiospores. Other modes of transmission include ingestion of the spore or inoculation of conidia from wounds or trauma.
- Nosocomial outbreaks of infections can also occur; however, these are quite rare. Nosocomial infections are associated with contaminated bandages, medical equipment, and ventilation.
- The mode of transmission of the fungi from one individual to the other depends on the site of infection and the severity of infection.
- Rhinocerebral mucormycosis transmits mostly via the inhalation of spores or droplets, whereas cutaneous mucormycosis transmits via close personal contact.
Pathogenesis of Mucormycosis
- The pathogenesis of mucormycosis begins with the inhalation or ingestion of spores from the environment.
- The entry of the spores into healthy individuals results in phagocytosis of the spores with the help of polymorphonuclear phagocytes.
- The persistence of the fungi and their growth is facilitated by defects in the phagocytic activity of the immune cells.
- Conditions like hyperglycemia and acidosis affect chemotaxis and phagocytic killing by the immune cells.
- Fungi like Rhizopus secrete the enzyme ketone reductase that supports the growth of fungi in acidic and glucose-rich environments like ketoacidosis.
- The increased virulence in the fungal species results in inherent resistance in these species to human phagocytes.
- Similarly, iron metabolism also plays an important role in the pathogenesis of mucormycosis. Different factors in the fungal species like the iron permeases, rhizoferrin, etc., help in the transition of ferric into soluble ferrous.
- The presence of iron in the serum further supports the growth and survival of the species in the human body.
- The fungi then slowly make their way into the bloodstream by invading blood vessels with resultant thrombosis and tissue necrosis.
- The host-pathogen interaction further results in extensive angioinvasion with ischemic necrosis and tissue damage.
- The movement of the organisms through endothelial cells and the extracellular matrix is the most critical step in the pathogenesis of fungal species like R. oryzae.
- The binding of the organism to the host endothelial cells results in endocytosis of the organism, which damages the endothelial cells. It has been recently understood that glucose-regulated protein (GRP78) acts as a receptor to mediate the penetration and damage of these cells.
- Since mucormycosis can occur due to a number of fungal species, the exact mechanism of disease or pathogenesis might not be the same for all species.
- Besides, the dissemination of the organism to a different part of the body can result in different forms of mucormycosis.
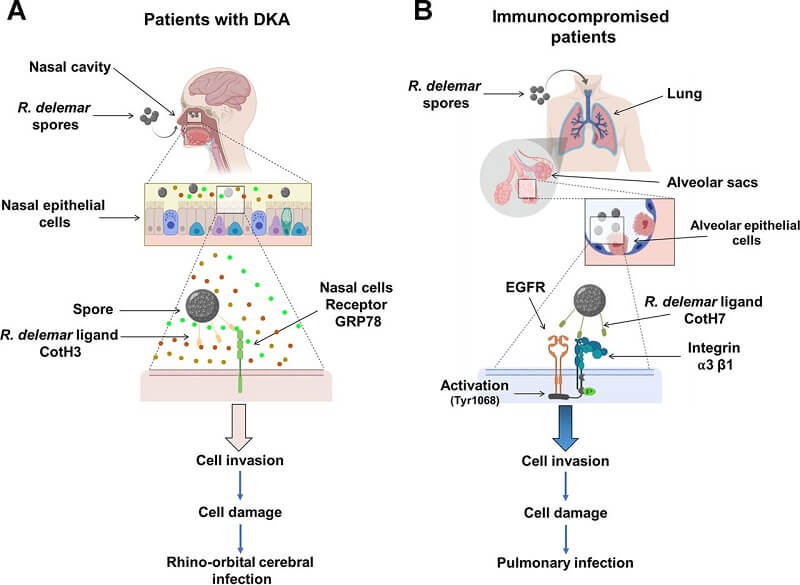
Figure: A diagram showing the molecular pathogenesis of the two main manifestations of mucormycosis. (A) R. delemar inhaled spores are trapped in the sinus cavities of patients with DKA due to the overexpression of GRP78 on nasal epithelial cells, and the interaction with fungal CotH3 results in rhinoorbital/cerebral mucormycosis. Colored circles represent elevated levels of glucose, iron, and ketone bodies. (B) In immunosuppressed patients, inhaled spores reach the alveoli and bind to integrin α3β1 via fungal CotH7, thereby triggering activation of EGFR and subsequent invasion and pulmonary infection. Image Source: mBio, American Society for Microbiology.
Types or Forms of Mucormycosis (Clinical Manifestations)
The clinical manifestations of the infections depend on the site of infection by the organisms as well as the mode of transmission of the organisms. The following are some of the types of mucormycosis that are observed in immunocompromised humans.
1. Rhinocerebral (Sinus and Brain) mucormycosis
- Rhinocerebral mucormycosis is a condition caused by filamentous fungi of the order Mucorales, which affect organs like the paranasal sinuses, nose, and brain.
- The disease is most acute, but it can become chronic as the fungus grows rapidly and aggressively.
- Rhinocerebral mucormycosis is the most common form of mucormycosis, and the prevalence of the infections depends on the occurrence of the different high-risk populations.
- The infection begins in the nasal cavity and slowly moves to the adjacent paranasal sinuses. The fungi then attached themselves to the surface of the sinus and began reproducing as the humid condition of the nose facilitates growth and invasion of the organism.
- The initial condition of the infection is associated with the formation of the fungal ball in the maxillary sinus with no bone erosion.
- The condition proliferates further depending on the duration of infection, host immunity, and severity of the condition.
- The progression of the diseases continues as a result of different virulence factors. It is initiated by the invasion of blood vessels and damage to the endothelial cells resulting in ischemia and tissue necrosis.
- The invasion of the brain and orbits of the brain is the result of the invasion of the sphenopalatine and internal maxillary arteries.
- Rhinocerebral mucormycosis occurs more commonly in diabetic patients with diabetic ketoacidosis and hyperglycemia.
- The early diagnosis of the infection is impeded by the nonspecific symptoms of the disease. Commonly observed symptoms include one-sided headache behind the eyes and lethargy.
- It is then followed by facial pain, numbness, nasal discharge, and sinusitis, along with convulsions, altered mental condition, and gait.
2. Pulmonary (Lung) mucormycosis
- Pulmonary mucormycosis is an uncommon form of mucormycosis but can result in life-threatening opportunistic infections.
- It is the second most common mucormycosis infection accounting for about 25% of total mucormycosis infections.
- The infection is more frequent in immunocompromised patients with transplants and hematological malignancies.
- Pulmonary mucormycosis has a high mortality rate of 40-70%, especially in cases with rapid local progression and angioinvasion.
- The infection proceeds from the entry of the organism via inhalation. The organism reaches the lung spaces where it adheres to the endothelial cells to result in tissue damage.
- The extent of damage and progression of the infection depends on the immune status of the individual and the underlying conditions.
- The diagnosis of the disease is based on intrapulmonary imaging with lobar and segmental consolidation.
- The increased mortality associated with the infection is the result of multifocal pneumonia with bilateral consolidation of the lungs. Single or multiple nodules can also be observed in some cases.
- The high mortality also results from delays in diagnosis, poor host response, and limited therapy available for the infection.
- Other common characteristics of the disease include fever, hemoptysis, and tissue infarction.
- The causative agent of pulmonary mucormycosis is mostly Rhizopus spp. followed by Mucor and Rhizomucor species.
3. Gastrointestinal mucormycosis
- Gastrointestinal mucormycosis is very rare and is observed only in about 2 to 11% of the total cases of mucormycosis.
- The organs involved in the infection are the stomach and intestine, but in some cases, the infection can spread to other regions of the intestinal tract.
- The infections are mostly mild, but in some cases, these can be fatal. The infection begins with the ingestion of spores with food or other substances that finally make way into the gastrointestinal tract.
- Aggressive antifungal treatments and medical therapy with surgeries can be used as a method of treatment.
4. Cutaneous (Skin) mucormycosis
- Cutaneous mucormycosis is also a form of mucormycosis either as a localized infection or dissemination disease.
- Cutaneous mucormycosis results from the entry of the pathogen through trauma or cuts on the skin as a result of surgery, natural disaster, or inoculation of soil and other contaminated sources.
- The infection can spread quite rapidly on the skin to inner layers like the subcutaneous layer, fascia, and bone.
- The predominant species involved in cutaneous mucormycosis are Apophysomyces and Saksenaea, both of which are common soil saprophytes.
- Cutaneous mucormycosis can be classified as primary and secondary mucormycosis, where the primary infections include infections where the organism infects the individual via direct inoculation. Secondary mucormycosis involves the dissemination of organisms from other locations, commonly a rhinocerebral infection.
- The most commonly affected areas in the case of cutaneous mucormycosis are legs and arms, including other rare cases in the scalp, face, back, thorax, breast, neck, and groin.
- Primary infections are characterized by lesions that are indurated plaques that eventually become erythematous.
- Other manifestations include tender nodules, swollen and scaly plaques with purpuric lesions.
- Primary infections can be nosocomial infections, in which case the erythema and tenderness rapidly progress to necrosis.
- Secondary infections result from the spread of rhinocerebral infection, and these are more common than primary infections.
- The infection begins with sinusitis which then progresses with the formation of a necrotic eschar.
- Other symptoms include fever, periorbital cellulitis, edema, and proptosis with later intracranial involvement.
5. Disseminated mucormycosis
- Disseminated mucormycosis is the rarest form of mucormycosis that is usually only observed in neutropenic patients with hematologic tumors or post-transplant patients.
- The cases are quite rare but have an extremely high mortality rate of about 90% as the infection tends to be invasive.
- The high rate of dissemination associated with mucormycosis is due to the tendency of invading endothelial cells within the vascular system.
- Dissemination of infections can occur in organs like the lung, pancreas, brain, and spleen, which mild to severe infections in all or some of the regions.
- The condition only occurs in severely immunocompromised individuals who have received deferoxamine.
- The symptoms and presentations associated with disseminated mucormycosis consist of nonspecific manifestations which result in delayed diagnosis and further invasion.
- The direct inoculation of the fungi is a common mode of transmission where the fungi can infect cutaneous, subcutaneous, fat muscles, and skeletal tissues.
- In severe cases, the organism can even reach deep organs and result in localized infections at multiple sites.
Laboratory Diagnosis of Mucormycosis
- The diagnosis of mucormycosis infections requires a high degree of suspicion, recognition of host factors, and proper assessment of different clinical manifestations.
- Early diagnosis of these infections can be taken as a possible method of prevention of severe conditions and mortality.
- Since the infections can occur in practically all organs, a syndrome-oriented approach to diagnosis is not very effective.
- The following are some of the modes of laboratory diagnosis of mucormycosis;
Microscopic Examination
- The first step in the diagnosis of mucormycosis is the microscopic examination of different clinical specimens.
- The species belonging to the order Morales have nonseptate or pauci-septate hyphae with variable width.
- Stains like Periodic acid Schiff or Grocott-Gromori’ methenamine silver can be used used to highlight the fungal hyphae and observe the morphology clearly.
- Invasive infections can be characterized by angioinvasion that can be observed in terms of the increased number of phagocytic cells like neutrophils and other granulocytes.
- Even if direct examination of clinical specimens is a quick and simple method, the histopathological examination cannot allow the differentiation of some species.
Serology
- Serological tests like ELIZA, immunoblots, and immunodiffusion tests can be performed for the diagnosis of mucormycosis with variable success.
- Mucorales-specific antibodies can be detected by the enzyme-linked immunospot assay to diagnose invasive mucormycosis.
- The process is, however, not very common as the antibodies specific to different fungal species are difficult to find and develop.
Molecular Assays
- Molecular assays including PCR, restriction fragment length polymorphism, and DNA sequencing provide the most reliable form of diagnosis of Mucorales.
- Most of the molecular-based diagnostic tools use an internal transcribed spacer or the 18S rRNA genes.
- The methods are still quite new, and there haven’t been enough data to specify the sensitivity and specificity of the tests.
- Molecular diagnosis with the blood and serum samples has been proposed and shown promising results.
Treatment of Mucormycosis
- The successful treatment of mucormycosis is based on a multi-step approach which requires reversal of underlying conditions, early administration of antifungal agents at optimal dosages, and removal of all infected tissues.
- In patients with uncontrolled diabetes and suspected mucormycosis, rapid correction of metabolic abnormalities is a must.
- The use of corticosteroids and other immunosuppressive drugs should be tapered to the lowest dose possible.
- The success of the treatment of mucormycosis depends on the early diagnosis of the disease with prompt initiation of therapeutic interventions.
- Mucorales can be resistant to most antifungal agents like voriconazole, but Amphotericin B is the most effective drug for most Mucorales.
- Other drugs include Posaconazole, Isavuconazole, itraconazole, and terbinafine with different degrees of activity against Mucorales.
- The medicinal therapy has to be introduced immediately once the infection is suspected due to the potential for the rapid spread of the infection.
- The optimal dose for these antifungal drugs is still in question; however, some guidelines have been proposed to provide the appropriate dose and concentration of these drugs.
- The prevalence of intravenous and tablet forms of these drugs has increased bioavailability and drug exposure.
- Other forms of treatment include surgeries when needed. Removal of not just the necrotic tissues but also surrounding infected healthy tissues might be required.
- Surgery is often required in rhinocerebral mucormycosis and soft tissue infections. Surgeries might also be helpful in the single localized pulmonary lesion.
- Other forms of medical therapy include the use of hyperbaric oxygen to prepare a more oxygen-rich environment and the administration of cytokines along with antifungal agents.
Prevention and Control of Mucormycosis
- The high mortality rate of these infections indicates the need for early intervention with immunocompromised individuals.
- It is important that the patients are aware of the infections and their presentations so that they can make an early visit to the hospital.
- Prevention and control of these infections are based on the early diagnosis of the disease and the maintenance of a proper immune system.
- Individuals at risk with different underlying conditions should be careful about any possible symptoms and other conditions.
- It has been recommended that the patients take appropriate drugs assigned for their underlying conditions in order to maintain their health.
- The control of the disease can also be made by the use of masks in areas that might contain the spores the causative agents.
- It is imperative to maintain a healthy diet and appropriate lifestyle in order to prevent severe cases of infection.
Mucormycosis and COVID-19
- Mucormycosis has been increasingly observed as a form of secondary fungal infection in COVID 19 patients.
- The most common form of mucormycosis in COVID 19 patients is pulmonary mucormycosis, closely followed by rhinocerebral mucormycosis.
- The incidence of mucormycosis with COVID 19 isn’t unusual as the disease tends to affect the immune status of the patients, resulting in increased chances of mucormycosis.
- Similarly, glucocorticoids and remdesivir are some of the only drugs that have been beneficial in COVID 19; however, the use of glucocorticoids can increase the risk of secondary infections.
- The use of concurrent immunomodulatory drugs and the immune dysregulation as a result of the viral infection further add to the increased risk of the infections.
- The correlation between COVID 19 and mucormycosis has still not been recognized completely due to the underdiagnosis of these infections.
- Some of the factors that help prevent the infections from turning severe include the control of hyperglycemia with early treatment with appropriate antifungal agents.
References
- Hernández JL, Buckley CJ. Mucormycosis. [Updated 2020 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544364/
- Bhandari J, Thada PK, Nagalli S. Rhinocerebral Mucormycosis. [Updated 2020 Nov 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559288/
- Ibrahim, Ashraf S et al. “Pathogenesis of mucormycosis.” Clinical infectious diseases : an official publication of the Infectious Diseases Society of America vol. 54 Suppl 1,Suppl 1 (2012): S16-22. doi:10.1093/cid/cir865
- Camara-Lemarroy, Carlos Rodrigo et al. “Clinical features and outcome of mucormycosis.” Interdisciplinary perspectives on infectious diseases vol. 2014 (2014): 562610. doi:10.1155/2014/562610
- Skiada, A et al. “Challenges in the diagnosis and treatment of mucormycosis.” Medical mycology vol. 56,suppl_1 (2018): 93-101. doi:10.1093/mmy/myx101
- Skiada, Anna et al. “Epidemiology and Diagnosis of Mucormycosis: An Update.” Journal of fungi (Basel, Switzerland) vol. 6,4 265. 2 Nov. 2020, doi:10.3390/jof6040265
- Reid G, Lynch JP 3rd, Fishbein MC, Clark NM. Mucormycosis. Semin Respir Crit Care Med. 2020 Feb;41(1):99-114. doi: 10.1055/s-0039-3401992. Epub 2020 Jan 30. PMID: 32000287.
- Hoffmann, K et al. “The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies.” Persoonia vol. 30 (2013): 57-76. doi:10.3767/003158513X666259
- Giulia Morace, Elisa Borghi, “Invasive Mold Infections: Virulence and Pathogenesis of Mucorales“, International Journal of Microbiology, vol. 2012, Article ID 349278, 5 pages, 2012. https://doi.org/10.1155/2012/349278
- Hassan MIA, Voigt K. Pathogenicity patterns of mucormycosis: epidemiology, interaction with immune cells and virulence factors. Med Mycol. 2019 Apr 1;57(Supplement_2):S245-S256. doi: 10.1093/mmy/myz011. PMID: 30816980; PMCID: PMC6394756.
- Lee FYW, Mossad SB, Adal KA. Pulmonary Mucormycosis: The Last 30 Years. Arch Intern Med. 1999;159(12):1301–1309. doi:10.1001/archinte.159.12.1301
- Fernandez, Juan F et al. “Pulmonary mucormycosis: what is the best strategy for therapy?.” Respiratory care vol. 58,5 (2013): e60-3. doi:10.4187/respcare.02106
- Castrejón-Pérez, Ana Daniela et al. “Cutaneous mucormycosis.” Anais brasileiros de dermatologia vol. 92,3 (2017): 304-311. doi:10.1590/abd1806-4841.20176614
- Sarrami, Amir Hossein et al. “Fatal disseminated mucormycosis in an immunocompotent patient: a case report and literature review.” International journal of preventive medicine vol. 4,12 (2013): 1468-71.
- Chen, Q., Chen, K., Qian, S., Wu, S., Xu, L., Huang, X. … Wang, X. (2019). Disseminated mucormycosis with cerebellum involvement due to Rhizomucor pusillus in a patient with multiple myeloma and secondary myelodysplastic syndrome: A case report. Experimental and Therapeutic Medicine, 18, 4076-4080. https://doi.org/10.3892/etm.2019.8065

This information is really simplified and easy to understand. I am grateful.