Mass Spectrometry (MS) is an analytical chemistry technique that helps identify the amount and type of chemicals present in a sample by measuring the mass-to-charge ratio and abundance of gas-phase ions.
In this instrumental technique, the sample is converted to rapidly moving positive ions by electron bombardment and charged particles are separated according to their masses.
A mass spectrum is a plot of relative abundance against the ratio of mass/charge (m/e).
These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical structures of molecules and other chemical compounds.
Interesting Science Videos
Principle of Mass Spectrometry (MS)
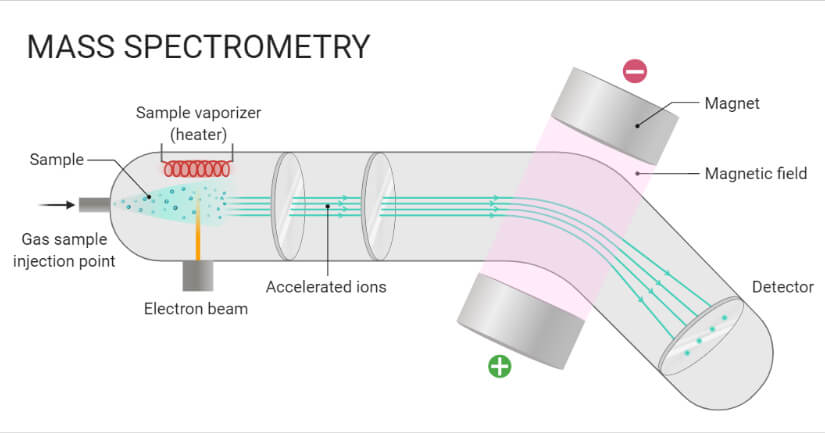
- In this technique, molecules are bombarded with a beam of energetic electrons.
- The molecules are ionized and broken up into many fragments, some of which are positive ions. Each kind of ion has a particular ratio of mass to charge, i.e. m/e ratio (value).
- For most ions, the charge is one, and thus, the m/e ratio is simply the molecular mass of the ion.
- The ions pass through magnetic and electric fields to reach the detector where they are detected and signals are recorded to give mass spectra.
Working of Mass Spectrometry (MS)
- In a typical procedure, a sample, which may be solid, liquid, or gas, is ionized, for example by bombarding it with electrons.
- This may cause some of the sample’s molecules to break into charged fragments. These ions are then separated according to their mass-to-charge ratio, typically by accelerating them and subjecting them to an electric or magnetic field:
- Ions of the same mass-to-charge ratio will undergo the same amount of deflection.
- The ions are detected by a mechanism capable of detecting charged particles, such as an electron multiplier. Results are displayed as spectra of the relative abundance of detected ions as a function of the mass-to-charge ratio.
- The atoms or molecules in the sample can be identified by correlating known masses (e.g. an entire molecule) to the identified masses or through a characteristic fragmentation pattern.
Instrumentation and Steps of Mass Spectrometry (MS)
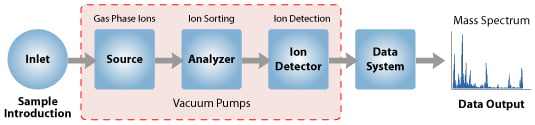
A. Sample Inlet
- A sample stored in the large reservoir from which molecules reach the ionization chamber at low pressure in a steady stream by a pinhole called “Molecular leak”.
B. Ionization
- Atoms are ionized by knocking one or more electrons off to give positive ions by bombardment with a stream of electrons. Most of the positive ions formed will carry a charge of +1.
- Ionization can be achieved by :
- Electron Ionization (EI-MS)
- Chemical Ionization (CI-MS)
- Desorption Technique (FAB)
C. Acceleration
- Ions are accelerated so that they all have the same kinetic energy.
- Positive ions pass through 3 slits with voltage in decreasing order.
- Middle slit carries intermediate and finals at zero volts.
D. Deflection
- Ions are deflected by a magnetic field due to differences in their masses.
- The lighter the mass, the more they are deflected.
- It also depends upon the no. of +ve charge an ion is carrying; the more +ve charge, the more it will be deflected.
E. Detection
- The beam of ions passing through the mass analyzer is detected by a detector on the basis of the m/e ratio.
- When an ion hits the metal box, the charge is neutralized by an electron jumping from the metal onto the ion.
- Types of analyzers:
- Magnetic sector mass analyzers
- Double focussing analyzers
- Quadrupole mass analysers
- Time of Flight analyzers (TOF)
- Ion trap analyzer
- Ion cyclotron analyser
Applications of Mass Spectrometry (MS)
- Environmental monitoring and analysis (soil, water, and air pollutants, water quality, etc.)
- Geochemistry – age determination, soil, and rock composition, oil and gas surveying
- Chemical and Petrochemical industry – Quality control
- Identify structures of biomolecules, such as carbohydrates, nucleic acids
- Sequence biopolymers such as proteins and oligosaccharides
- Determination of the molecular mass of peptides, proteins, and oligonucleotides.
- Monitoring gases in patients’ breath during surgery.
- Identification of drug abuse and metabolites of drugs of abuse in blood, urine, and saliva.
- Analyses of aerosol particles.
- Determination of pesticides residues in food.
References and Sources
- https://www.thermofisher.com/au/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-mass-spectrometry.html
- https://www.slideshare.net/akshukumarsharma/mass-spectroscopy 55382941
- http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch13/ch13-ms.html
- https://en.wikipedia.org/wiki/Mass_spectrometry
- https://www.chemguide.co.uk/analysis/masspec/howitworks.html
- https://www.slideshare.net/solairajananant/mass-spectrometry-38534267
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/massspec/masspec1.htm

It was more usefull then a full 1 hour video lacture ❤️
Thank you so much , all the notes provided by you are so helpful .
Thank you so much , all the notes provided you are so helpful .
Very nice 🙂💯
About TOFSIMD
V nice 👍
I want gcms,and ion chromatography notes
We will soon have notes on these topics, Thanks,
you are well come.I need details of mass spectroscopy