- Major Histocompatibility Complex (MHC) is a part of the genome of all vertebrates that code for molecules which are important in immune recognition.
- In humans, the MHC is a cluster of genes located on chromosome 6 which code for MHC proteins also called Human Leukocyte Antigen (HLA).
- MHC proteins are a set of cell surface proteins essential for the acquired immune system to recognize foreign molecules which in turn determines histocompatibility.
- The main function of MHC molecules is to bind to peptide antigens and display them on the cell surface for recognition by appropriate T-cells.
- Among the many genes in the human MHC, those that encode the class I, class II, and class III MHC proteins are considered important.
- MHC class II molecules are a class of major histocompatibility complex (MHC) molecules normally found only on antigen-presenting cells which are important in initiating immune responses.
- MHC Class II proteins are encoded by the genes of HLA-D region of the genome in humans.
- Unlike class I proteins, they have a restricted tissue distribution and are chiefly found on macrophages, dendritic cells, B cells, and other Antigen Presenting Cells (APCs) only.
- However, their expression on other cells (eg, endothelial cells or epithelial cells) is induced by IFN-γ.
- The antigens presented by class II peptides are derived from extracellular proteins and not endogenous antigens as in MHC class I.
- Class II MHC molecules have β1 and β2 subunits and thus can be recognized by CD4 co-receptors.
- These MHC Class II molecules sample extracellular peptides mainly extracellular pathogens and by interacting with immune cells like the T helper cell (TCD4+) regulate how T cells respond to an infection.
Interesting Science Videos
Structure of Major Histocompatibility Complex II
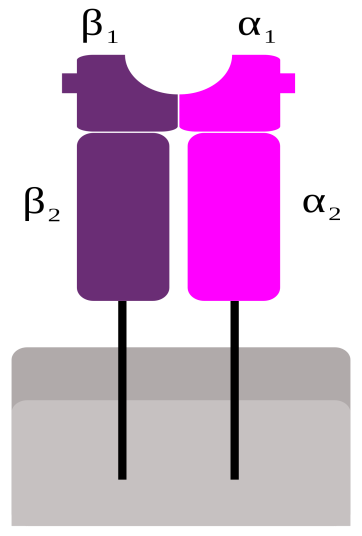
- MHC-II molecules are dimers consisting of a 133 KDa α-chain and 28KDa β-chain which are associated by non-covalent interactions.
- Both the α-chain and β-chain are made up of two domains – α1 and α2 and β1 and β2 respectively.
- They are membrane bound glycoprotein that contains external domains, a transmembrane segment and a cytoplasmic tail.
- An open ended groove formed between α-chain and β-chain at the proximal end serves as the peptide biding cleft which can bind antigenic peptide.
Mechanism of Major Histocompatibility Complex II
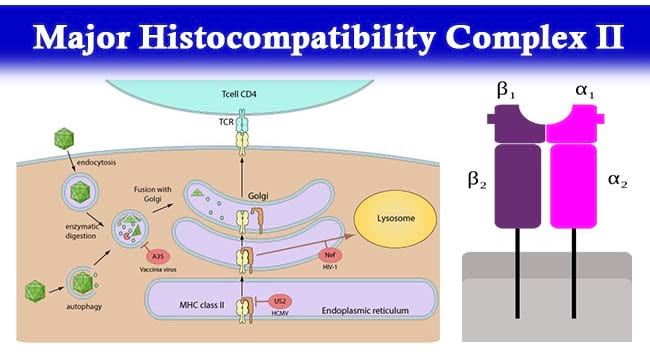
- MHC class II molecules present antigens of exogenous origin to CD4+ T cells.
- Phagocytes such as macrophages and immature dendritic cells take up entities by phagocytosis into phagosomes which fuse with lysosomes and the acidic enzymes cleave the uptaken protein into many different peptides.
- During synthesis of class II MHC, the molecules are transported from the endoplasmic reticulum (ER) via the Golgi to endosomal compartments. The α and β chains produced are complexed with a special polypeptide known as the invariant chain (Ii). The Ii prevents endogenous peptides from binding to the groove of MHC class II molecules.
- After removal of Ii in the acidic endosomal compartments, peptides are able to bind to the MHC groove.
- A particular peptide exhibiting immunodominance loads onto MHC class II molecules.
- Peptide-loaded MHC class II molecules are then transported to the membrane surface for antigen presentation.
- The peptide:MHC class II complex is then recognized by the cognate T cell receptor (TCR) of helper T cells.
Functions of Major Histocompatibility Complex II
- The TCR–peptide: MHC class II engagement is crucial to the induction and regulation of adaptive immunity by selecting the mature CD4+ T cell repertoire in the thymus and activating these lymphocytes in the periphery.
- The secure attachment to the MHC molecule with the presented peptide ensure stable peptide binding which enhance T cell recognition of the antigen, T cell recruitment, and a proper immune response.
- Since they sample and present antigens from exogenous sources, MHC class II molecules are critical for the initiation of the antigen-specific immune response.
Reference
- Brooks, G. F., Jawetz, E., Melnick, J. L., & Adelberg, E. A. (2010). Jawetz, Melnick, & Adelberg’s Medical Microbiology. New York: McGraw Hill Medical.
- Chelbi, S., Dang, A., & Guarda, G. (2017). Emerging Major Histocompatibility Complex Class I -Realted Functions of NLRC5. Advances in Immunology , 133, 89-119.
- Kindt, T., Goldsby, R., Osborne, B., Kuby, J. and Kuby, J. (2007). Kuby immunology. New York: W.H. Freeman.
