- Major Histocompatibility Complex (MHC) is a part of the genome of all vertebrates that code for molecules which are important in immune recognition.
- In humans, the MHC is a cluster of genes located on chromosome 6 which code for MHC proteins also called Human Leukocyte Antigen (HLA).
- MHC proteins are a set of cell surface proteins essential for the acquired immune system to recognize foreign molecules which in turn determines histocompatibility.
- The main function of MHC molecules is to bind to peptide antigens and display them on the cell surface for recognition by appropriate T-cells.
- Among the many genes in the human MHC, those that encode the class I, class II, and class III MHC proteins are considered important.
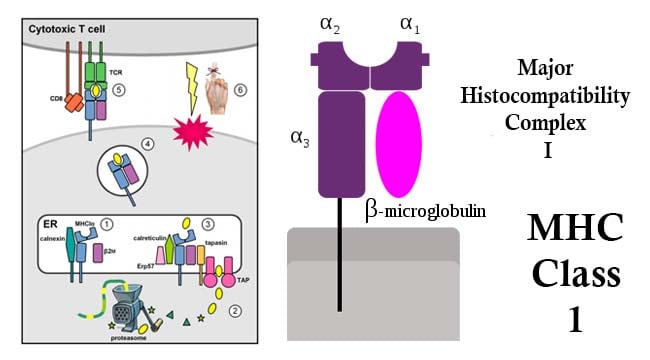
- MHC class I proteins are encoded by the HLA-A, HLA-B, and HLA-C genes encoding HLA-A, HLA-B, and HLA-C molecules respectively.
- Class I molecules are found on virtually all nucleated cells in the body including platelets. Key exceptions are observed on cells in the retina and brain and the non- nucleated red blood cells.
- They are recognized by CD8 co-receptors through the MHC Class I β2 subunit.
- These MHC Class I molecules sample peptides generated within the cell and signal the cell’s physiological state to effector cells of the immune system, particularly CD8+ T lymphocyte.
Interesting Science Videos
Structure of Major Histocompatibility Complex I
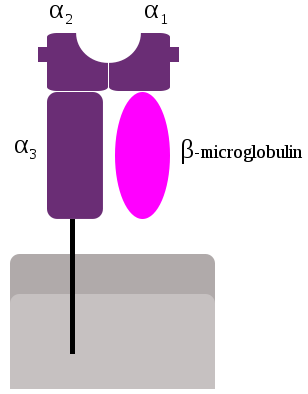
- Class-I MHC is a glycoprotein molecule containing a 45KDa α-chain associated non-covalentely with a 12KDa β2 microglobulin molecule.
- The α chain composed of three domains—α1, α2, and α3.
- The α1 rests upon β2 microglobulin while the α3 domain is transmembrane anchoring the MHC class I molecule to the cell membrane.
- The peptide-binding groove present in the central region of the α1/α2 heterodimer helds the peptide being presented.
Mechanism of Major Histocompatibility Complex I
- MHC class I glycoproteins present antigens of endogenous origin to TCRs of CD8+ T cells.
- Endogenous peptides derive from degradation of intracellular proteins, including viral or tumor antigens in infected or transformed cells, through the proteasome.
- Degradation products translocate from the cytoplasm to the endoplasmatic reticulum (ER) where they are loaded on MHC class I molecules via the peptide-loading complex that includes the ER transporter associated with antigen processing (TAP1/2), tapasin, the oxidoreductase ERp57, and the chaperone protein calreticulin.
- Cellular components involved in the presentation of endogenous antigens, from proteasome subunits to the peptide-loading complex, are collectively referred to as antigen-processing machinery (APM).
- CD8+ T lymphocytes express CD8 receptors, in addition to the T-cell receptors (TCR).
- When a Cytotoxic T cell CD8 receptor docks to a MHC class I molecule and the TCR fits the epitope within the MHC class I molecule, the CD8+ T lymphocytes triggers the cell to undergo programmed cell death by apoptosis.
- This helps mediate cellular immunity which is the primary means to address intracellular pathogens, such as viruses and some bacteria.
Functions of Major Histocompatibility Complex I
-
Antigen Processing and Presentation
Nucleated cell normally present peptides, mostly self peptides derived from protein turnover and defective ribosomal products. Also, during viral infection, intracellular microorganism infection, or cancerous transformation, such proteins degraded inside the cell by proteasomes are also loaded onto MHC class I molecules and displayed on the cell surface.
-
Transplant Rejection
During transplant of an organ or stem cells, MHC molecules themselves act as antigens and can provoke immune response in the recipient causing transplant rejection. Since, the MHC variation in the human population is high and no two individuals except identical twins express the same MHC molecules, they can mediate transplant rejection.
Reference
- Brooks, G. F., Jawetz, E., Melnick, J. L., & Adelberg, E. A. (2010). Jawetz, Melnick, & Adelberg’s Medical Microbiology. New York: McGraw Hill Medical.
- Chelbi, S., Dang, A., & Guarda, G. (2017). Emerging Major Histocompatibility Complex Class I -Realted Functions of NLRC5. Advances in Immunology , 133, 89-119.
- Kindt, T., Goldsby, R., Osborne, B., Kuby, J. and Kuby, J. (2007). Kuby immunology. New York: W.H. Freeman.
- Major Histocompatibility Complex. In Wikipedia. Retrieved June 10, 2018, from https://en.wikipedia.org/wiki/Major_histocompatibility_complex

Good