Enzymes are biological catalysts. These are commonly proteins but also include RNA (ribozymes) molecules that catalyze chemical reactions by lowering the activation energy of a reaction. These are known to speed up the rate of a reaction millions of times faster than the reaction without enzymes. Nearly all biological reactions require enzymes to transform substrate into products. The substrate is the reactant molecule upon which enzymes act during a chemical reaction, and products are the substances formed as a result of a chemical reaction. A single reactant molecule can decompose to give multiple products. Similarly, two reactants can enter into a reaction to yield products. These are reusable even after the completion of the reaction. Chemical properties such as charge and pH are vital in enzymatic reactions.
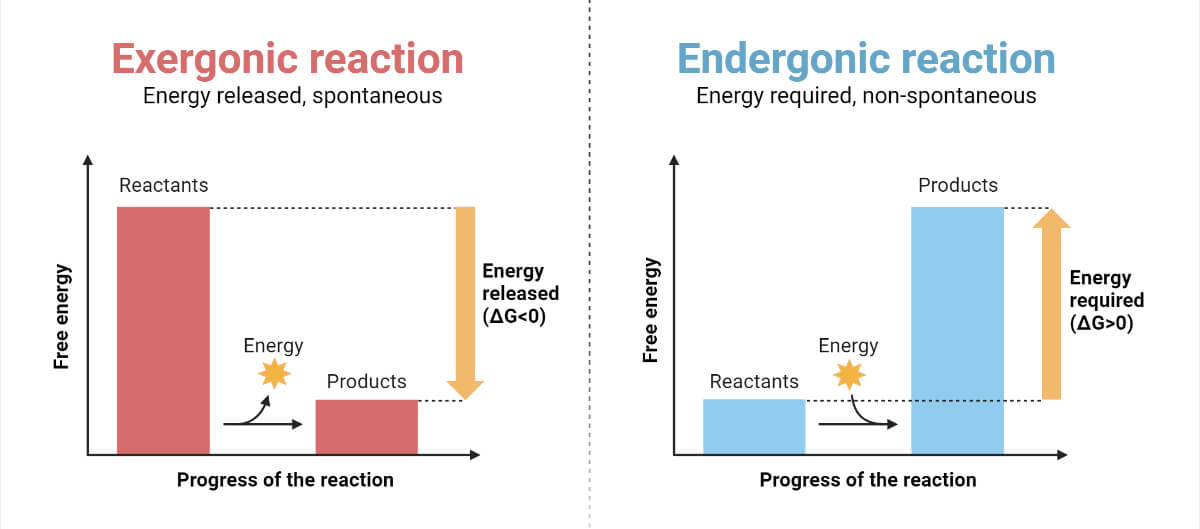
Binding between enzymes and reactant molecules takes place in such a way that chemical bond-breaking and bond-forming processes occur more readily. Meanwhile, no change in ∆the G value of a reaction takes place, thereby not altering the energy-releasing or energy-absorbing process of the reaction. However, it lowers the energy of the transition state, the topmost unstable state where the activated complex is formed from reactants that later give products.
Interesting Science Videos
Enzyme’s Active site and Substrate Specificity
Enzymes are relatively larger than the substrates, whose only a small fraction is involved in catalysis by reducing chemical activation energy, also known as the catalytic site, and the other portion for binding with the substrate and orienting them also known as the binding site. The catalytic site and binding site altogether form the active site of an enzyme. Usually, there are two active sites in an enzyme.
- The active site of enzymes is a cleft portion, composed of a small number of a unique combination of amino acid residues, usually three to four in number, which make up only ~10-20% of the volume of an enzyme.
- The remaining amino acids are used to maintain tertiary structure by proper scaffold folding through non-covalent interactions.
- Non-covalent interaction between enzyme and substrate in correct orientation favors their reaction. These interactions include hydrogen bonds, hydrophobic bonds, ionic interactions, and Van der Waal’s interactions.
- However, transient covalent bonds between enzymes and substrates are also formed during the time of reaction.
- Side chains of amino acids play an important role in highly specific three-dimensional conformation at the level of the active site. These are large or small, hydrophilic or hydrophobic, acidic or basic.
- The specific shape, size, and chemical behavior of enzymes are determined by the nature of amino acids and their 3D space in the active site.
Specificity is a distinctive feature of enzymes where they have a unique ability to choose an exact substrate from a group of similar chemical molecules. Their specificity towards their substrate varies to a different extent. These are of different types, namely: Bond specificity, Group specificity. Substrate specificity, Stereospecificity, Geometrical specificity, and Co-factor specificity.
Substrate specificity is also k/a absolute specificity for the enzyme’s specificity towards one substrate and one reaction. For e.g., Lactase acts on the B-1-4 glycosidic linkage of lactose to yield galactose and glucose. The restrictive nature of enzymes towards the choice of substrate can be attributed to the enzymatic activity of two oxidoreductase enzymes. Alcohol dehydrogenase uses its substrate alcohol while lactic acid dehydrogenase act on lactic acid. Although these two enzymes function with the mechanism of oxidation and reduction reaction, their substrates can’t be used interchangeably. This is because the different structure of each substrate prevents their fitting into the active site of the alternative enzyme.
In most cases, cofactors, the non-protein molecules, are required to ensure an efficient enzyme-facilitated chemical reaction. These function to bind with enzymes via either ionic interaction or covalent interactions. Metal ions (such as minerals) and co-enzymes (vitamin derivatives) are cofactors.
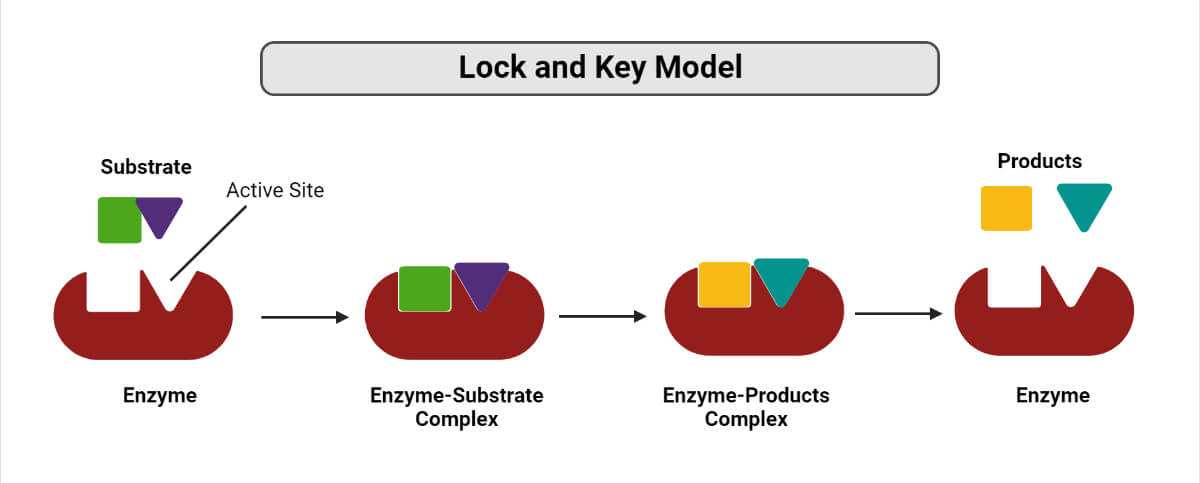
Lock and Key Model
A German scientist, Emil Fischer postulated the lock and key model in 1894 to explain the enzyme’s mode of action. Fischer’s theory hypothesized that enzymes exhibit a high degree of specificity towards the substrate. This model assumes that the active site of the enzyme and the substrate fit perfectly into one another such that each possesses specific predetermined complementary geometric shapes and sizes. Thus, the shape of the enzyme and substrate do not influence each other. This specificity is analog to the lock and key model, where the lock is the enzyme, and the key is the substrate. In certain circumstances, if a second substrate similar in shape and size to the primary substrate is made to bind to the enzyme, this second substrate also fits in the active site too.
How does Lock and Key Model work?
- Binding of the substrate(s) to the enzyme at their active site takes place, thereby forming an enzyme-substrate complex.
- Enzymes catalyze the chemical reaction to take place, which can either be a synthesis reaction (favors bond formation) or a decomposition reaction (favors bond breakage).
- As a result, the formation of one or more products takes place, and the enzymes are released for their reuse in the next reaction.
Limitations of Lock and Key Model
- It doesn’t explain how the enzyme-substrate complex is stabilized in the transition state.
- This model supposes the enzyme is a rigid structure whose shape does not change upon binding with a suitable substrate. However, this is not supported by recent research, which states that there is a change in conformation of the active site of the enzyme upon binding of substrate.
- It does not describe the condition for binding multiple substrates to the enzyme.
Later, it was found that enzyme specificity toward one substrate is not always true. Although there are enzymes that specifically bind with only one substrate, there are also enzymes that exhibit broad specificity towards different but similarly structured substrates, such as lipase that can bind to different types of lipids. Similarly, proteases such as trypsin and chymotrypsin can degrade multiple types of proteins. Thus, the lock and key model is flawed, and the induced fit model was introduced to give a more refined view of the mode of enzymatic action.
References
- Blanco, A., & Blanco, G. (2017). Medical Biochemistry. Academic Press.
https://www.khanacademy.org/science/ap-biology/cellular-energetics/enzyme-structure-and-catalysis/a/enzymes-and-the-active-site - https://www.biologyonline.com/dictionary/substrate-specificity
- https://www.britannica.com/science/protein/The-mechanism-of-enzymatic-action
- https://www.biologyonline.com/dictionary/lock-and-key-model
- https://study.com/learn/lesson/lock-key-model-vs-induced-fit-model.html
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/26%3A_Biomolecules-_Amino_Acids_Peptides_and_Proteins/26.11%3A_Enzymes_and_Coenzymes
- https://en.wikibooks.org/wiki/Structural_Biochemistry/Protein_function/Lock_and_Key
- https://ib.bioninja.com.au/higher-level/topic-8-metabolism-cell/untitled-6/models-of-action.html
