Interesting Science Videos
Structure of Japanese Encephalitis (JE) Virus
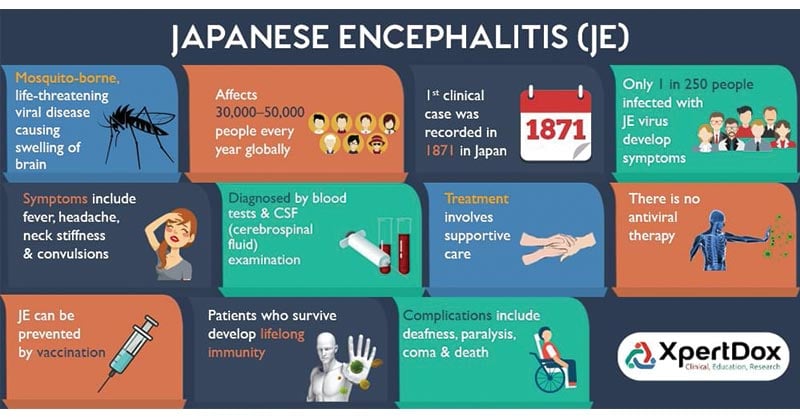
- Japanese encephalitis (JE) virus falls under the family Flaviviridae.
- The particles appear to be spherical, 50 nm in diameter, containing an electron dense core (about 30 nm diameter) surrounded by a lipid bilayer.
- Mature virions sediment between 170 and 210S, have a buoyant density of 1.19 to 1.23 g/mL.
- It has a small lipoprotein envelope surrounding a nucleocapsid comprising of the core protein.
- The nucleocapsid also encloses the single stranded RNA genome with positive polarity which is 11kb in length.
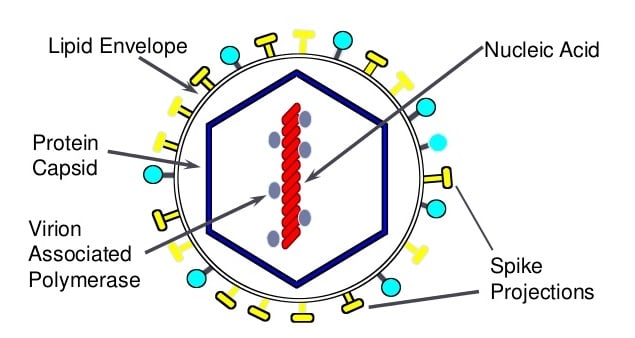
Genome of Japanese Encephalitis (JE) Virus
- The genome is monopartite, linear single stranded RNA genome with positive polarity.
- The ssRNA genome is 11kb in length.
- This RNA contains a 5′ cap (m7G5’ppp5’A) at the 5′ end and lacks a polyadenylate tail.
- Genomic RNA is the messenger RNA for translation of a single long open reading frame (ORF) as a large polyprotein.
- Surrounding the ORF are 5′ and 3′ noncoding regions (NCRs) of around 100 nucleotides (nt) and 400 to 700 nt, respectively.
- The genome is translated as a large polyprotein that is processed co- and posttranslationally by cellular proteases and a virally encoded serine protease into at least 10 discrete products.
- The N-terminal one quarter of the polyprotein encodes the structural proteins, and the remainder contains the nonstructural (NS) proteins, in the following order: C-prM-E-NS1-NS2A-NS3-NS4A-NS4B-NS5.
- Three viral proteins are associated with virions: the E (envelope), M (membrane), and C (capsid) proteins.
- The E protein (50kd) is the major surface protein of the viral particle, probably interacts with viral receptors, and mediates virus–cell membrane fusion.
- M protein is a small proteolytic fragment of prM protein (26kd), which is important for maturation of the virus into an infectious form.
- C protein (about 11 kd) is highly basic, consistent with its proposed role in forming a ribonucleoprotein complex with packaged genomic RNA.
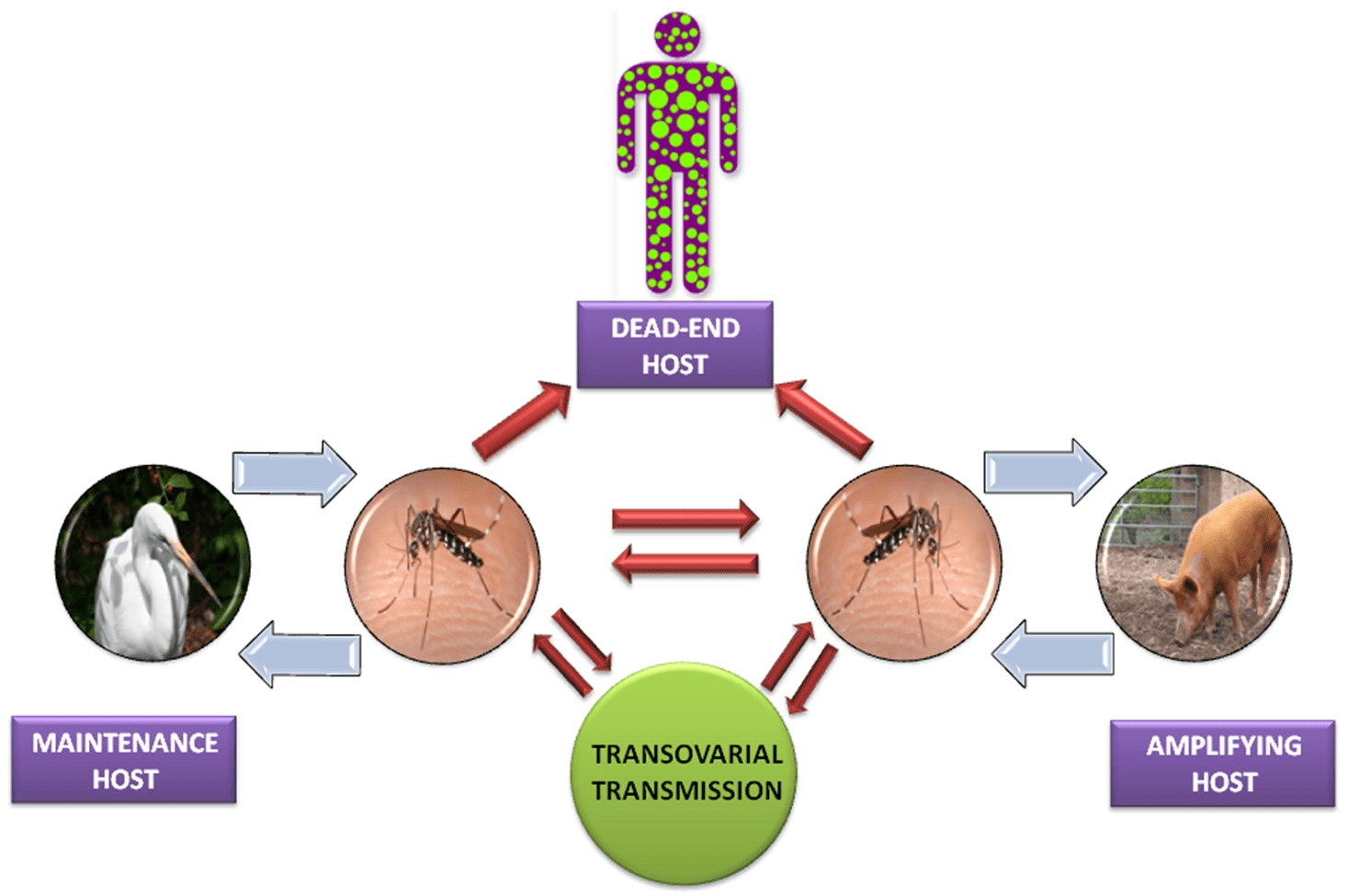
Epidemiology and Transmission of Japanese Encephalitis (JE) Virus
- JE virus is transmitted by the bite of Culex mosquitoes and transmitted naturally between wild and domestic birds and pigs.
- The virus is maintained in an enzootic cycle between mosquitoes and amplifying vertebrate hosts (mainly pigs).
- Most important one for human infection is Culex tritaeniorrhynchus, which breeds in pools of stagnant water.
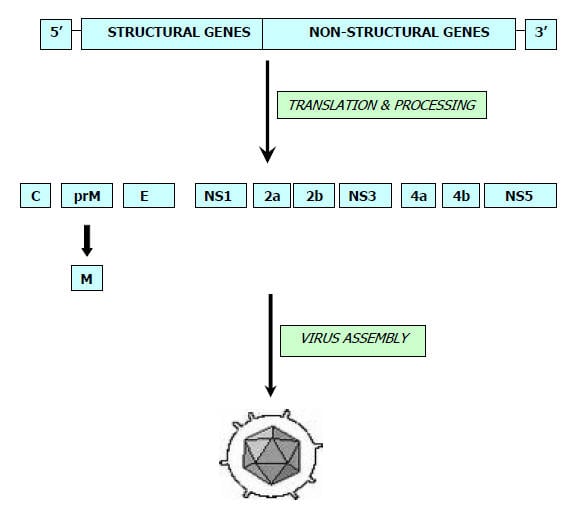
- The virus occurs in countries across Eastern, Southern Asia and Pacific.
- Humans become infected coincidentally when living or travelling in close proximity to animals and birds infected with JE.
- Human are considered as dead end host from which transmission doesn’t not normally occur.
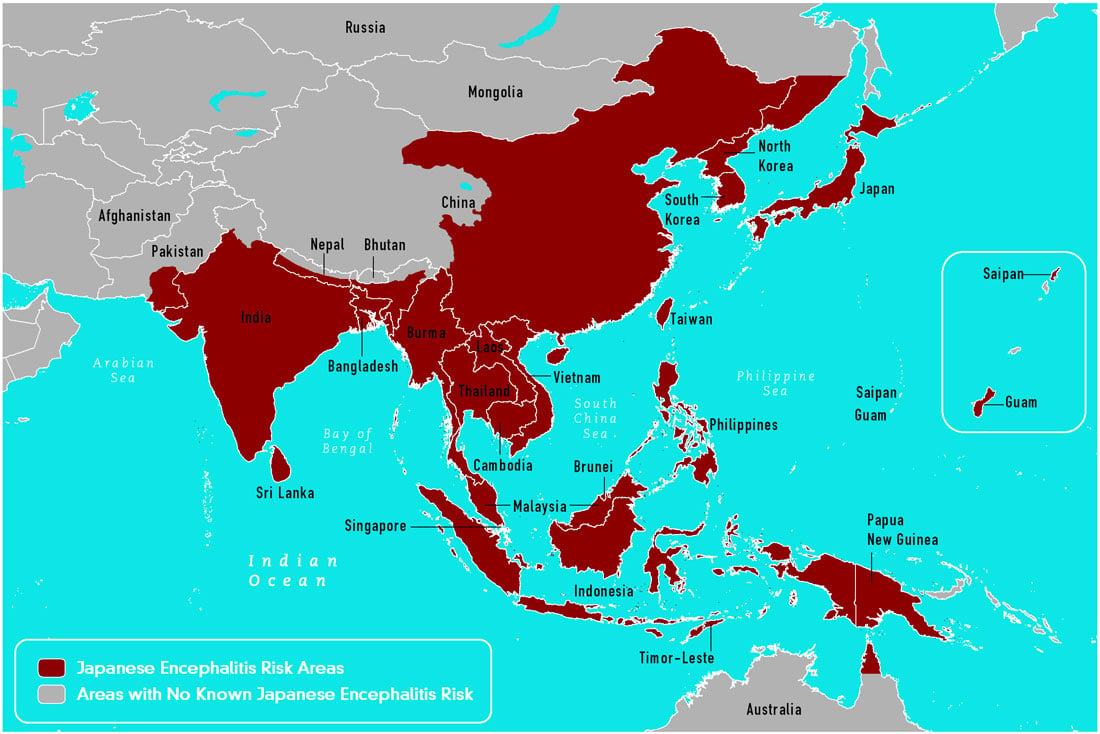
Source: CDC, 2015
- Two epidemiological patterns of JE are recognized:
- During summer month’s large epidemics occur in Northern areas which include-Northern Vietnam, Northern Thailand, Taiwan, China, Republic of Korea, Japan, Nepal, and northern India.
- In Southern areas like Southern Vietnam, Southern Thailand, Indonesia, Malaysia, Philippines, SriLanka and Southern India, JE tends to be endemic and cases occur sporadically throughout the year with the peak after the start of the rainy season.
Replication of Japanese Encephalitis (JE) Virus
- Attachment of the viral envelope protein E to host receptors mediates internalization into the host cell by clathrin-mediated endocytosis, or by apoptotic mimicry.
- Fusion of virus membrane with host endosomal membrane and results in release of RNA genome into the cytoplasm.
- The positive-sense genomic ssRNA is translated into a polyprotein, which is cleaved into all structural and non structural proteins to yield the replication proteins.
- Following translation and processing of the viral proteins, a viral replicase is assembled from NS proteins, the viral RNA, and presumably some host factors.
- Replication begins with the synthesis of a genome-length minus-strand RNA, which then serves as a template for the synthesis of additional plus-strand RNAs.
- Virus assembly occurs at the endoplasmic reticulum.
- The virion buds at the endoplasmic reticulum and is transported to the Golgi apparatus.
- The prM protein is cleaved in the Golgi, thereby maturing the virion which is fusion competent.
- Release of new virions by exocytosis.
Pathogenesis of Japanese Encephalitis (JE) Virus
- The portal of entry for the JE virus is through the bite of mosquito which contain virus.
- After the bite on skin the virus enter the Reticuloendothelial system (RES) and follows transient phase of viremia.
- After the transient viremia the virus invades the central nervous system.
- The virus enters the neuroparenchyma by crossing the capillary walls of brain and distributes itself in hypothalamus, hippocampus, substantia nigra and medulla oblongata regions of brain via vascular endothelial cells by the mechanism of endocytosis.
- The mechanism of endocytosis is either cholesterol or clathrin mediated pathway.
- Virus then replicates in neurons and matures in the neuronal secretory system.
- JE typically develops in patients after an incubation period of 5–15 days.
- It is possible that during this time, the virus resides and multiplies within host leukocytes, which act as carriers to the CNS.
- T lymphocytes and IgM play a major role in the recovery and clearance of the virus after infection.
- Besides neuronal cells, researchers have shown that astrocytes are also infected by JEV.
- Crossing the blood brain barrier is an important factor in the increased pathogenesis and clinical outcome of the neurotropic viral infection.
- Astrocytes, being a component of the blood–brain barrier, may help in the transmission of JEV from peripheral tissues to the cerebrospinal fluid.
- It is known that JEV causes neuronal cell death in two ways: direct neuronal killing , wherein viral multiplication within neuronal cells leads to cell death, and the indirect mode of killing, wherein massive inflammatory response causes an up-regulation of reactive oxygen species and cytokines such as tumor necrosis factor α (TNFα), which, in turn, causes neuronal death.
Clinical Manifestations of Japanese Encephalitis (JE) Virus
- The clinical picture of the Japanese encephalitis include three phases:
- Prodromal phase: The incubation period is 1-6 days and the characteristic features of disease condition include malaise, anorexia, headache, fever and vomiting. In children, diarrhea and abdominal pain may be prominent.
- Acute encephalitic phase: The incubation period is 7-13 days. The signs and symptoms includes photophobia, hyper excitability, focal and neurologic signs, muscular rigidity, dull, mask like face, tremulous eye movements, cranial nerve palsies, speech impairment ad stiff neck.
- Late convalescent phase: The incubation period is 14-15 days. The clinical picture demonstrated as mental impairment, neurological signs and symptoms, epilepsy, abnormal movements, and CNS injury.
- Common clinical laboratory findings include moderate leukocytosis, mild anemia, and hyponatremia.
- Cerebrospinal fluid (CSF) typically has a mild to moderate pleocytosis with a lymphocytic predominance, slightly elevated protein, and normal ratio of CSF to plasma glucose.
Laboratory Diagnosis of Japanese Encephalitis (JE) Virus
- Specimens: CSF, Blood, Plasma, Serum, Tissue(rare)
- Detection of JE virus–specific IgM by using a JE virus–specific IgM-capture ELISA on CSF or serum.
- JE virus–specific IgM can be measured in the CSF of most patients by 4 days after onset of symptoms and in serum by 7 days after onset.
- Plaque reduction neutralization tests can be performed to confirm the presence of JE virus–specific neutralizing antibodies and discriminate between cross-reacting antibodies from closely related flaviviruses.
- A greater 4-fold rise in JE virus– specific neutralizing antibodies between acute-and convalescent-phase serum specimens may be used to confirm recent infection.
- Detection of JE antigen in tissue by immunofluorescence or immunohistochemistry.
- Detection of JE virus genome in serum, plasma, blood, CSF or tissue by RT-PCR.
Treatment of Japanese Encephalitis (JE) Virus
- There is no specific antiviral treatment for JE.
- Therapy consists of supportive care and management of complications.
Prevention and Control of Japanese Encephalitis (JE) Virus
Vaccination
- One JE vaccine is licensed and available in the United States which is an inactivated Vero cell culture derived vaccine, Ixiaro.
- Other inactivated and live attenuated JE vaccines are manufactured and used in other countries.
- The vaccine is given as a 2-dose series, with the doses spaced 28 days apart and administered intramuscularly.
- The second dose should be given at least a week before travel.
- Children younger than 3 years of age get a smaller dose than patients who are 3 or older.
- A booster dose might be recommended for anyone 17 or older who was vaccinated more than a year ago and is still at risk of exposure.
- The Advisory Committee on Immunization Practices recommends JE vaccine for travelers who plan to spend ≥1 month in endemic areas during the JE virus transmission season which includes long-term travelers, recurrent travelers, or expatriates who will be based in urban areas but are likely to visit endemic rural or agricultural areas during a high-risk period of JE virus transmission.
- Early case detection and treatment.
- Use of Larvicides, insecticides, and ecofriendly methods such as using neem cakes and growing larvivorous fish in controlling mosquitoes in paddy fields.
- Vector control by reduction of breeding source for larvae, reduction of man-mosquito contact by wearing proper clothing to reduce mosquito bites and control of adult mosquitoes.