Interesting Science Videos
What is Infrared (IR) Spectroscopy?
Infrared (IR) spectroscopy or vibrational spectroscopy is an analytical technique that takes advantage of the vibrational transitions of a molecule.
It is one of the most common and widely used spectroscopic techniques employed mainly by inorganic and organic chemists due to its usefulness in determining the structures of compounds and identifying them.
The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) to produce an infrared spectrum.
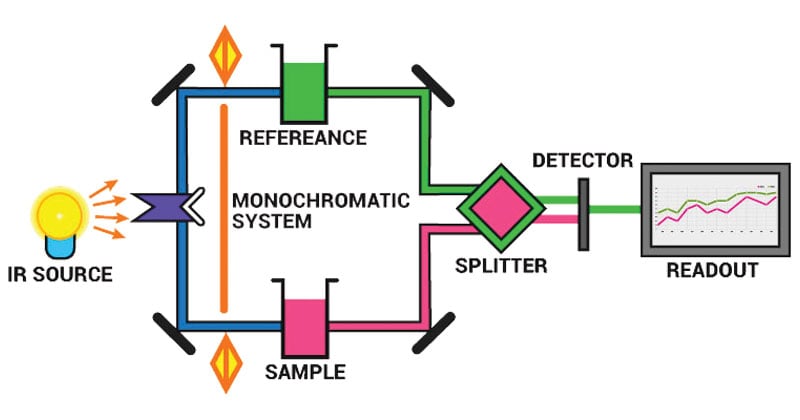
Image Source: BYJUS.
Principle of Infrared (IR) Spectroscopy
- Infrared Spectroscopy is the analysis of infrared light interacting with a molecule.
- The portion of the infrared region most useful for analysis of organic compounds have a wavelength range from 2,500 to 16,000 nm, with a corresponding frequency range from 1.9*1013 to 1.2*1014 Hz.
- Photon energies associated with this part of the infrared (from 1 to 15 kcal/mole) are not large enough to excite electrons, but may induce vibrational excitation of covalently bonded atoms and groups.
- It is known that in addition to the facile rotation of groups about single bonds, molecules experience a wide variety of vibrational motions, characteristic of their component atoms.
- Consequently, virtually all organic compounds will absorb infrared radiation that corresponds in energy to these vibrations.
- Infrared spectrometers, similar in principle to other spectrometer, permit chemists to obtain absorption spectra of compounds that are a unique reflection of their molecular structure.
- The fundamental measurement obtained in infrared spectroscopy is an infrared spectrum, which is a plot of measured infrared intensity versus wavelength (or frequency) of light.
- IR Spectroscopy measures the vibrations of atoms, and based on this it is possible to determine the functional groups.
- Generally, stronger bonds and light atoms will vibrate at a high stretching frequency (wavenumber).
Instrumentation of Infrared (IR) Spectroscopy
The main parts of the IR spectrometer are as follows:
- Radiation source
- Sample cells and sampling of substances
- Monochromators
- Detectors
- Recorder
A. IR radiation sources
IR instruments require a source of radiant energy which emits IR radiation which must be steady, intense enough for detection, and extend over the desired wavelength.
Various sources of IR radiations are as follows.
- Nernst glower
- Incandescent lamp
- Mercury arc
- Tungsten lamp
- Glober source
- Nichrome wire
B. Sample cells and sampling of substances
IR spectroscopy has been used for the characterization of solid, liquid, or gas samples.
i. Solid – Various techniques are used for preparing solid samples such as pressed pellet technique, solid run in solution, solid films, mull technique, etc.
ii. Liquid – Samples can be held using a liquid sample cell made of alkali halides. Aqueous solvents cannot be used as they will dissolve alkali halides. Only organic solvents like chloroform can be used.
iii. Gas– Sampling of gas is similar to the sampling of liquids.
C. Monochromators
- Various types of monochromators are prism, gratings and filters.
- Prisms are made of Potassium bromide, Sodium chloride or Caesium iodide.
- Filters are made up of Lithium Fluoride and Diffraction gratings are made up of alkali halides.
D. Detectors
- Detectors are used to measure the intensity of unabsorbed infrared radiation.
- Detectors like thermocouples, Bolometers, thermisters, Golay cell, and pyro-electric detectors are used.
E. Recorders
- Recorders are used to record the IR spectrum.
Applications of Infrared (IR) Spectroscopy
It has been of great significance to scientific researchers in many fields such as:
- Protein characterization
- Nanoscale semiconductor analysis and
- Space exploration.
- Analysis of gaseous, liquid or solid samples
- Identification of compounds
- Quantitative analysis
- Information regarding functional groups of molecules and constitution of molecules can be deduced from IR spectrum
- To know about interaction among molecules
Reference
- https://chem.libretexts.org
- https://en.wikipedia.org/wiki/Infrared_spectroscopy
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/infrared/infrared.htm
- http://www.wag.caltech.edu/home/jang/genchem/infrared.htm
- https://web.vscht.cz/~poustkaj/EN%20ASFA%20AU%20Koplik_Infrared_spectroscopy.pdf
- https://www.pharmatutor.org/pharma-analysis/analytical-aspects-of-infra-red spectroscopy-ir/instrumentation-of-ir-spectrophotometry

Most vital and very useful information about infrared spectroscopy.Kudos!
very helpful.
Is it possible to obtain permission to use the diagram of the infrared spectrophotomer on
https://microbenotes.com/infrared-ir-spectroscopy/
in a textbook entitled Organic Chemistry, an Acid-Base Approach, 3rd edition, to be published by Taylor and Francis in 2022.
Michael B. Smith (author) at michael.smith@uconn.edu
Thank yoiu
Hello Michael B. Smith, Thank you so much for your comment. The original source of the image is https://byjus.com/chemistry/infrared-spectroscopy/.
FTIR missing
Nice work very informative post keep doing like this..