Interesting Science Videos
Structure of Influenza A Virus
- Influenza A virus falls under the family Orthomyxoviridae.
- Influenza A virus particles are usually spherical and about 80- 120 nm in diameter.
- It is an enveloped virus and the envelope contains two glycoproteins, hemagglutinin (HA) and neuraminidase (NA), the membrane (M2) protein and is internally lined by the matrix (M1) protein.
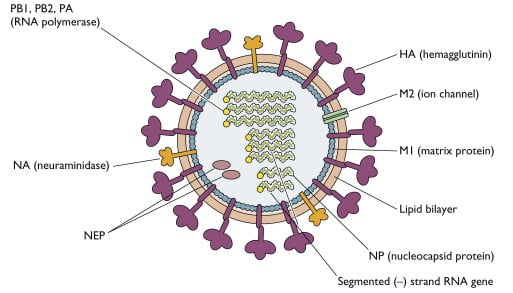
- The HA, so-called because the virus agglutinates certain species of erythrocyte, is about 10 nm in length and consists of trimers of identical glycoprotein subunits, each consisting of two polypeptide chains, HA1 and HA2 joined by a linkage site that may be a single basic amino acid, usually arginine, or a string of basic amino acids.
- The Neuramindase (NA) on the other hand is tetramer which facilitates release of virus particles from infected cell surfaces during budding process and helps prevent self aggregation of virions by removing sialic acid residues from viral gycloproteins via sialidase enzyme.
- They have a helical nucleocapsid comprising eight segments of single-stranded RNA of negative sense.
- Influenza virus particles contain nine different structural proteins.
- The nucleoprotein (NP) associates with the viral RNA to form a ribonucleoprotein (RNP) structure 9 nm in diameter that assumes a helical configuration and forms the viral nucleocapsid.
- Three large proteins (PB2, PB1, and PA) are bound to the viral RNP and are responsible for RNA transcription and replication.
- The matrix (M1) protein, which forms a shell underneath the viral lipid envelope, is important in particle morphogenesis and is a major component of the virion.
- M2, on the other hand, makes membrane channel protein and facilitate uncoating.
- The non structural protein NS is further divided into two parts NS1 that inhibits cellular mRNA translation and NS2 which is responsible for nuclear export of viral ribonuclear protein.
Genome of Influenza A Virus
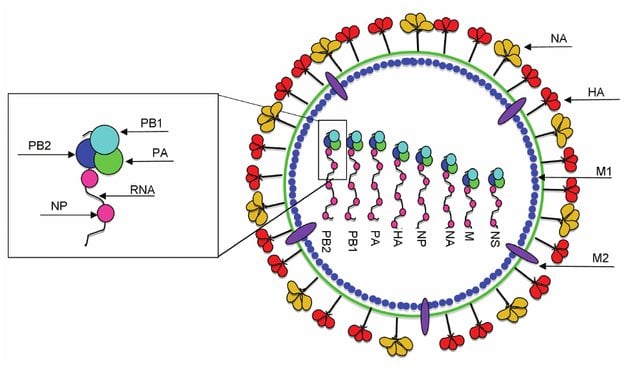
- The genome of influenza A virus is segmented ssRNA(-) linear genome, encapsidated by nucleoprotein (NP).
- It contains 8 segments coding for proteins.
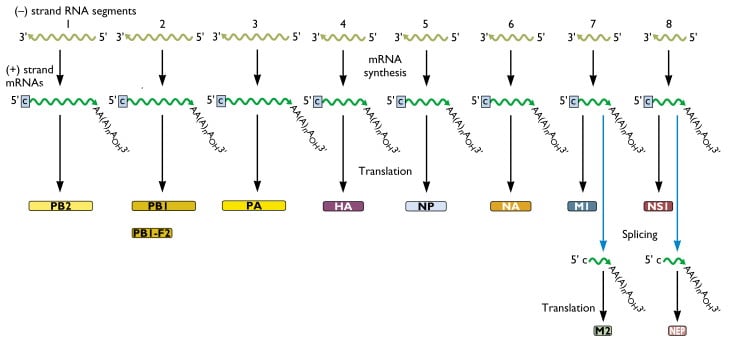
- Segments size range from 890 to 2,341nt and genomic size is 13.5Kb.
- All the proteins are encoded on separate segments, with the exception of the nonstructural proteins (NS1 and NS2) and the M1 and M2 proteins, which are transcribed from one segment each.
- The eight segments of genome comprise of PB2, PB1, PA, HA, NP, NA, Matrix protein (M1 and M2), non structural proteins (NS1 and NS2).
- PB2, PB1 and PA are the polymerase proteins, have transcription activity and convert the negative sense mRNA to positive sense.
- Haemagglutination (HA) is responsible for viral attachment, Nucleoprotein (NP) helps in making nucleocapsid and Neuraminidase (NA) cleaves sialic acid and promotes viral spread.
- Matrix protein M1 makes inner lining of envelope and promotes assesmbly and M2 make membrane channel protein facilitating uncoating.
- Non structural protein, NS1 reduce interferon reaction and inhibits RNA splicing.
- On the other hand, NS2 is required for the nuclear export of viral RNP.
Epidemiology of Influenza A Virus
- Influenza A viruses are classified into subtypes based on the antigenic difference of the major membrane glycoproteins HA and NA.
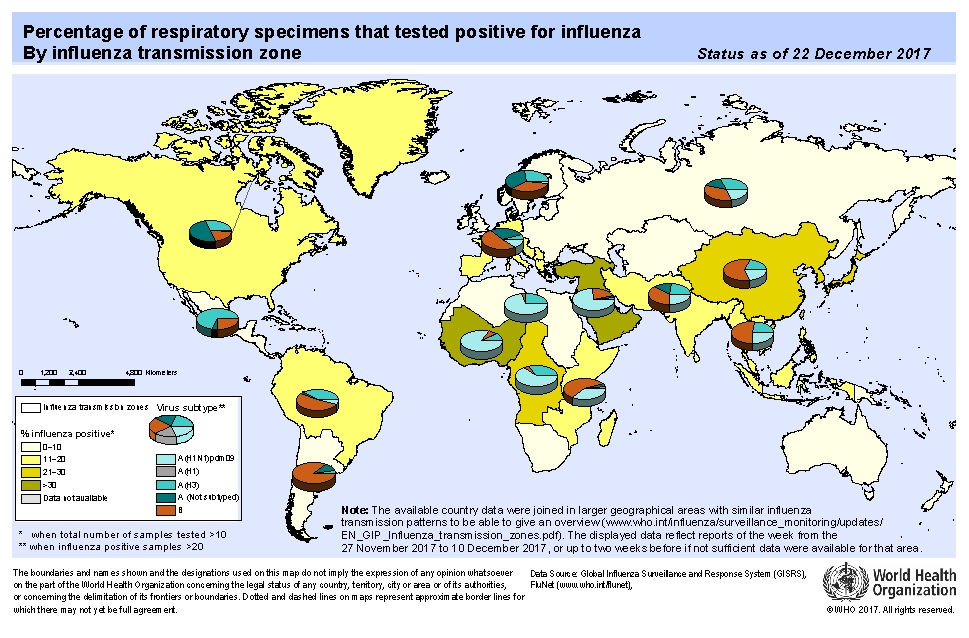
- Currently 18 HA subtypes and 11 NA subtypes are found.
- The different combinations have been recovered from birds, animal and humans.
- Four HA (H1, H2, H3, H5) and two NA (N1, N2) subtypes have been recovered from humans.
- The virus infects multiple species including humans, birds, swine, horses, seals, mink and whales with birds being the primary reservoir.
- Influenza viruses occur worldwide and cause annual outbreaks of variable intensity.
- It is estimated that annual epidemics of seasonal influenza cause 3–5 million cases of severe illness and 250,000–500,000 deaths worldwide.
- The economic impact of influenza A outbreaks is significant because of the morbidity associated with infections.
- Pandemics occurred in 1918, 1957 and 1968 with the emergence of H1N1 Spanish influenza, H2N2 and H3N2 respectively, and most recently in 2009, with the emergence of H1N1 from swine (H1N1 2009pdm) into the human population.
- The great pandemic of 1918– 1919 was particularly severe, killing 20–40 million people as it spread over a few years.
- Influenza A virus was first isolated from throat washing of patient by Smith Andrews and Laidlaw in 1933.
- Major pandemics are associated with antigenic shifts – when the viral HA or NA (or both) is changed.
- Antigenic shift results from the acquisition of a complete new RNA segment 4 and/or 6, either as a result of reassortment or infection of humans with an animal virus.
- Two influenza A subtypes have been circulating concurrently, namely Influenza A H3N2 and Influenza A.
- Epidemics occurring regularly in winter months between pandemics are associated with genetic drift in the HA antigen.
Replication in nucleus of Influenza A Virus
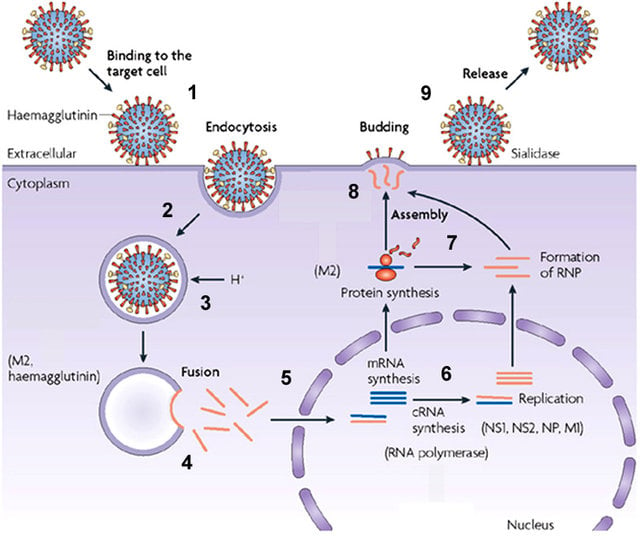
- Virus attaches to sialic acid receptor through HA protein and is endocytosedby clathrin mediated endocytosis into the host cell.
- After receptor-mediated endocytosis, the viral ribonucleoprotein complexes are released into the cytoplasm and transported to the nucleus, where replication and transcription take place.
- Messenger RNAs are exported to the cytoplasm for translation.
- Early viral proteins required for replication and transcription, including nucleoprotein (NP) and a polymerase protein (PB), are transported back to the nucleus.
- RNA polymerase activity of the PB1 protein synthesizes positive single-stranded RNA (ssRNA) from genomic negative single-stranded RNA (–ssRNA) molecules.
- These +ssRNA templates are copied by the RNA polymerase activity of the PB1 protein.
- Some of these new genome segments serve as templates for the synthesis of more viral mRNA.
- Viral mRNA molecules transcribed from some genome segments encode structural proteins such as hemagglutinin (HA) and neuraminidase (NA).
- These messages are translated by endoplasmic reticulum-associated ribosomes and delivered to the cell membrane.
- Viral genome segments are packaged as progeny virions and bud from the host cell.
Pathogenesis of Influenza A Virus
- Influenza virus spreads from person to person by airborne droplets or by contact with contaminated hands or surfaces.
- A person becomes infected when they inhale microdroplets containing virus.
- The respiratory tract, upper and lower respiratory tract have sialic acid to which HA portion of virus bind.
- A few cells of respiratory epithelium are infected if deposited virus particles avoid removal by the cough reflex and escape neutralization by preexisting specific immunoglobulin A (IgA) antibodies or inactivation by nonspecific inhibitors inthe mucous secretions.
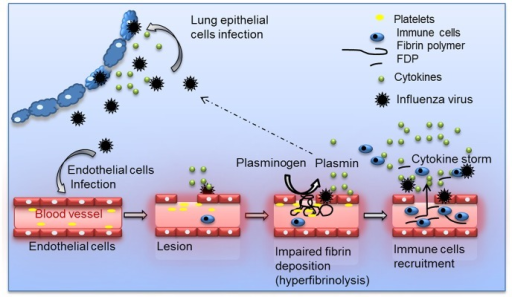
- The replication of virus takes place in nucleus and progeny virions are soon produced and spread to adjacent cells.
- Viral NA lowers the viscosity of the mucous film in the respiratory tract, laying bare the cellular surface receptors and promoting the spread of virus-containing fluid to lower portions of the tract.
- Within a short time, many cells in the respiratory tract are infected and eventually killed.
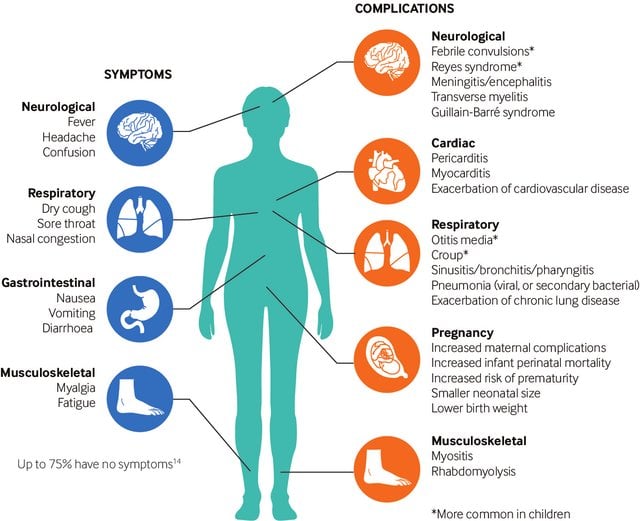
- Additional flu like symptoms which include sneezing, fever, chills, muscle ache, headache and fatigue occur.
- The incubation period from exposure to virus and the onset of illness varies from 1 day to 4 days, depending on the size of the viral dose and the immune status of the host.
- Viral shedding starts the day preceding onset of symptoms, peaks within 24 hours, remains elevated for 1–2 days, and then declines over the next 5 days.
- Interferon is detectable in respiratory secretions about 1 day after viral shedding begins.
- If the virus spreads to the lower respiratory tract, the infection can cause severe desquamation (shedding) of bronchial or alveolar epithelium down to a single-cell basal layer or to the basement membrane.
- Viral damage to the respiratory tract epithelium lowers its resistance to secondary bacterial invaders especially staphylococci, streptococci, and Haemophilus influenza.
- Influenza infection leads to an inflammatory cell response of the mucosal membrane, which consists primarily of monocytes and lymphocytes and few neutrophils.
- Submucosal edema is present.
- Lung tissue may reveal hyaline membrane disease, alveolar emphysema, and necrosis of the alveolar walls.
- T-cell responses are important in aspects of recovery and immunopathogenesis, but antibody, including vaccine-induced antibody can prevent disease.
- Protection against reinfection is primarily associated with the development of antibodies to HA, but antibodies to NA are also protective.
- The antibody response is specific for each strain of influenza, but the cell-mediated immune response is more general and is capable of reacting to influenza strains of the same type.
Clinical manifestations of Influenza A Virus
- The typical incubation period for influenza is 1- 4 days (average: 2 days).
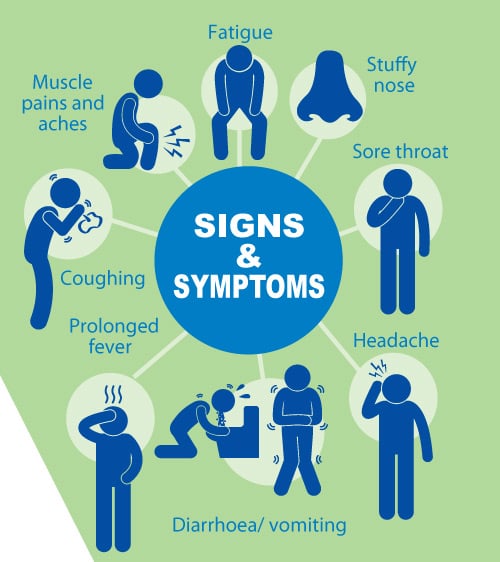
- Uncomplicated influenza illness is characterized by the abrupt onset of constitutional and respiratory signs and symptoms (e.g., fever, myalgia, headache, malaise, nonproductive cough, sore throat, and rhinitis).
- Among children, otitis media, nausea, and vomiting also are commonly reported with influenza illness.
- Uncomplicated influenza illness typically resolves after 3—7 days for the majority of persons, although cough and malaise can persist for >2 weeks.
- Influenza virus infections can cause primary influenza, viral pneumonia; exacerbate underlying medical conditions (e.g., pulmonary or cardiac disease); lead to secondary bacterial pneumonia, sinusitis, or otitis media; or contribute to coinfections with other viral or bacterial pathogens.
- Influenza virus infection also has been uncommonly associated with encephalopathy, transverse myelitis, myositis, myocarditis, pericarditis, and Reye’s
Complications of Influenza A Virus
- Tracheobronchitis and bronchiolitis– A small proportion of patients develop more severe respiratory symptoms where rales and rhonchi are heard but the chest is radiologically clear.
- Pneumonia
- Primary viral pneumonia or a secondary bacterial pneumonia may develop.
- Primary viral pneumonia is relatively uncommon, but cases have been demonstrated in many influenza epidemics.
- Secondary bacterial pneumonia is more common than primary viral pneumonia.
- Secondary bacterial pneumonia
- It usually occurs late in the course of disease, after a period of improvement has been observed for the acute disease.
- The symptoms and signs are that of a typical bacterial pneumonia.
- S. aureus is most commonly involved although S. pneumoniae and H. influenzae may be found.
- Infection of cells by influenza A requires cleavage of the virus haemagglutinin by proteases, and some strains of aureus produces such enzymes and hence promote infection by damaging to the healthy respiratory epithelium.
- Myositis and myoglobinuria– In addition to myalgia, which is characteristic of acute influenza infection, clinical myositis and myoglobinuria may occur.
- Reye’s syndrome
- Reye’s syndrome is characterized by encephalopathy and fatty liver degeneration.
- The disease has a 50% mortality amongst hospitalized cases and had been associated with several viruses; such as influenza A and B, Coxsackie B5, echovirus, HSV, VZV, CMV and adenovirus.
- Other complications
- Influenza infections have been implicated in acute viral encephalitis and Guillain-Barre syndrome.
- Influenza A was also associated with the cot death syndrome.
Laboratory diagnosis of Influenza A Virus
- Specimen– nasopharyngeal aspirate, throat swab, nasal swab, tracheal aspirate, bronchoalveolar lavage (BAL), sputum
- Virus isolation
- Throat swabs, NPA and nasal washings may be used for virus isolation.
- The specimen may be inoculated in embryonated eggs or tissue culture.
- 10-12 day embryonated eggs are used for virus isolation.
- The specimen is inoculated into the amniotic cavity.
- The virus replicates in the cells of the amniotic membrane and large quantities are released back into the amniotic fluid.
- After 2-3 days incubation, virus in the amniotic fluid can be detected by adding aliquots of harvested amniotic fluid to chick, guinea pig, or human erythrocytes.
- Pathological specimens can be inoculated on to tissue cultures of kidney, chicks or a variety of other species, Rhesus monkey cells being the most sensitive one.
- Although no CPE is produced, newly produced virus can be recognized by haemadsorption using the cells in the tissue culture, and haemagglutination using the culture medium which contains free virus particles.
- Occasionally influenza A produce a CPE in MDCK (Madin Darby Canine Kidney) cells.
- Influenza viruses isolated from embryonated eggs or tissue culture can be identified by serological or molecular methods.
- Rapid diagnosis
- Cells from pathological specimens may be examined for the presence of Influenza A antigen by Indirect Immunofluoresence (IFA).
- Enzyme immunoassay (EIA) are available for detection of viral antigen which are highly sensitive and specific.
- RT- PCR assays for the detection of influenza RNA have also been developed.
- Serology
- Serological test is based on the demonstration of a rise in antibody to the infecting virus.
- Complement fixation test (CFT) is the most common method used using type specific soluble antigen, however the specificity is low.
- Haemagglutination inhibition (HAI) test is more specific in comparison to CFT. However, both the tests requires a 4-fold or greater rise in antibody titre for detection.
- A more precise method for measuring antibody is by Single Radial Haemolysis (SRH) and is more sensitive than CFT and HAI test.
Treatment of Influenza A Virus
- Amantidine and Rimantidine are M2 ion channel inhibitors, thus preventing the pH changes that precede the membrane fusion step essential for nucleocapsid release.
- Zanamavir- potent inhibitor of neuraminidase and administered by inhalation.
- Oseltamavir- inhibitor of neuraminidase and administered orally.
Prevention of Influenza A Virus
- Vaccination is the most effective measure for reducing the impact of influenza.
- In view of the changing antigenic characteristics of the virus, new vaccines are constantly required and should contain H and N components of prevalent strain.
Types of vaccine of Influenza A Virus
- Killed vaccines
- The vaccines are prepared from virus grown in embryonated eggs and then chemically inactivated using formalin or beta propiolactone.
- The quantity of HA is standardized in each vaccine dose (~15 μg of antigen), but the quantity of NA is not standardized because it is more labile under purification and storage conditions.
- The vaccine is conventionally formulated in aqueous or saline suspension.
- The vaccine is administered by the subcutaneous or intramuscular route.
- Split virus vaccines
- Split vaccines were prepared from inactivated particles disrupted with detergents.
- These vaccines have been shown to induce fewer side effects in the vaccinees and are just immunogenic as whole virus vaccine.
- Subunit virus vaccines
- Subunit vaccines have been prepared by the combination of HA and NA antigens.
- These are used in aqueous suspension or may be absorbed to carriers such as alhydrogel.
- Live attenuated vaccines
- Normal methods for attenuation, such as repeated passages and temperature adaptation require a long period to complete, and probably too long for the vaccine to become available for immunization against the current influenza strain.
- A trivalent live attenuated influenza vaccine is administered as a single dose intranasal
- The trivalent vaccine consists of reassortant for the HA and NA gene segments of the desired influenza strains, with a master donor virus that is cold adapted to optimum growth at 25° C.
Control of Influenza A Virus
- Housing domestic poultry in shelters to avoid contact with over-flying migrating birds.
- Eliminating wild bird markets.
- Segregating different species of birds in markets.
- Housing aquatic birds and domestic poultry separately.
- Slaughtering domestic flocks infected with highly pathogenic influenza A viruses.

Thanks for existing thats it … Very grateful 😆
very helpful notes. thanks
influenza virus genome is outstanding write.