- Antibodies, or ‘immunoglobulins’, are glycoproteins that bind antigens with high specificity and affinity.
- In humans there are five chemically and physically distinct classes of antibodies (IgG, IgA, IgM, IgD, IgE).
- Immunoglobulin G (IgG), the most abundant type of antibody, is found in all body fluids and protects against bacterial and viral infections.
- It represents approximately 75% of serum antibodies in humans and thus the most common type of antibody found in the circulation.
- It has a serum concentration of 10 to 16 mg/mL and also considered as the major immunoglobulin in extravascular spaces.
- IgG antibodies elicit responses such as Antibody-Dependent Cellular Cytotoxicity (ADCC), Antibody-Dependent Cellular Phagocytosis (ADCP) and Complement-Mediated Cytotoxicity (CMC).
Interesting Science Videos
Structure of Immunoglobulin G (IgG)
- IgG antibodies are large monomeric molecules of about 150 kDa with a tetrameric quaternary structure.
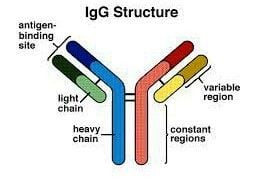
- An IgG antibody comprises of heavy and light chains. It possesses the basic monomeric “H2L2” structure consisting of 2 identical Heavy (H) and 2 identical Light (L) chains.
- Its H-chain type is gamma (γ heavy chains) about 50 kDa in weight and each H chain is paired with an L chain of about 25 kDa.
- The two heavy chains are linked to each other and to a light chain each by disulfide bonds.
- The resulting tetramer has two identical halves, which together form the Y-like shape.
- Each end of the fork contains an identical antigen binding site. Thus, each IgG has two antigen binding sites.
- The heavy chain components are CH1, CH2, CH3, hinge and the VH (variable region of heavy chain) and light chains consist of CL (Constant region of light chain) and the κ or λ chains.
- Peculiarly, the Fc regions of IgGs bear a highly conserved N-glycosylation site.
IgG Subclasses
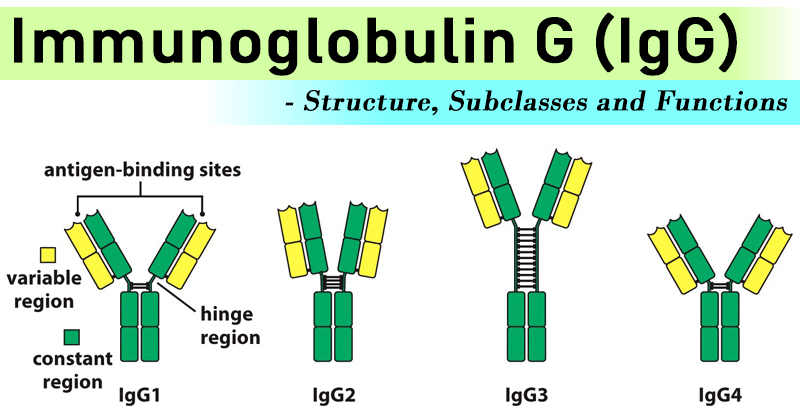
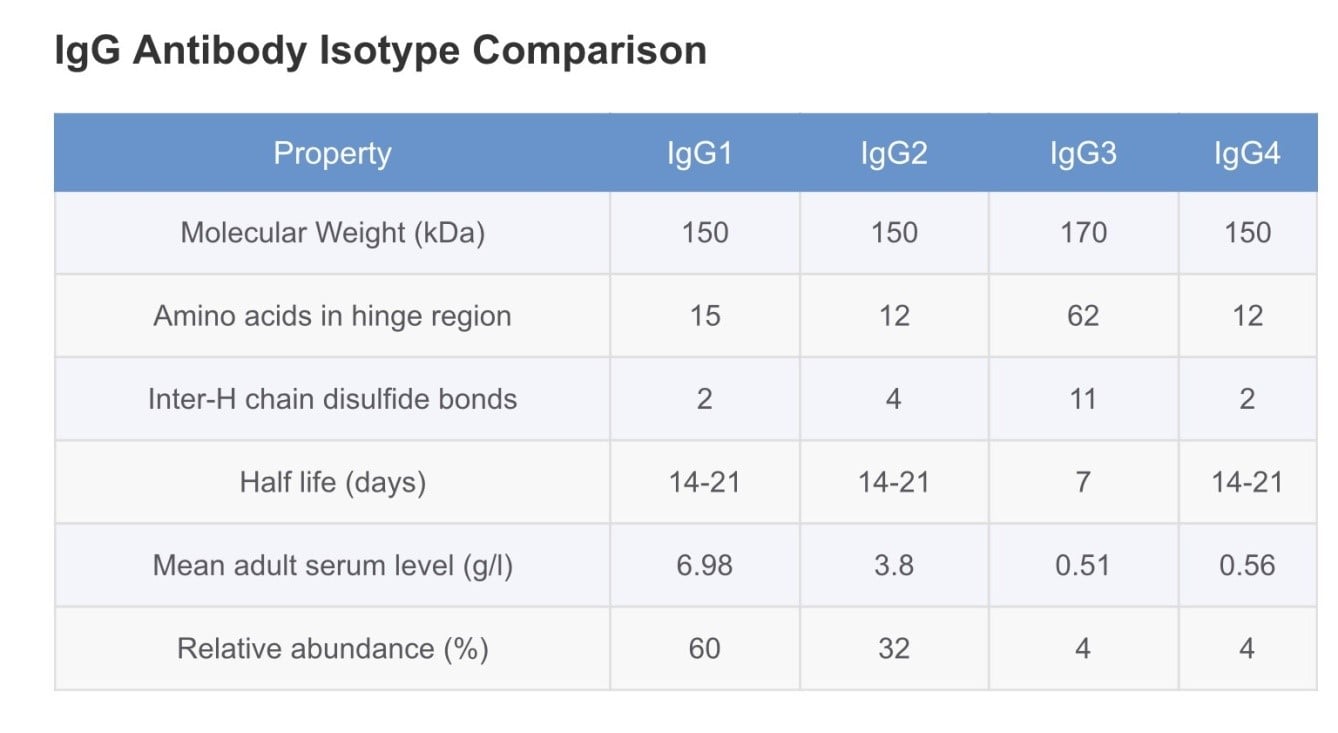
- There are four IgG subclasses (IgG1, 2, 3, and 4) in humans, named in order of their abundance in serum (IgG1 being the most abundant).
- Subclasses IgG1, IgG2, IgG3, and IgG4 are differentiated on the basis of the size of the hinge region, position of interchain disulfide bonds, and molecular weight. The subclasses also differ in their ability to activate complement.
IgG1
It comprises 60 to 65% of the total main subclass IgG, and predominantly responsible for the thymus-mediated immune response against proteins and polypeptide antigens. It is also involved in opsonization and activation of the complement cascade. A deficiency in IgG1 isotype is typically a sign of a hypogammaglobulinemia.
IgG2
It comprises 20 to 25% of the main subclass and is the prevalent immune response against carbohydrate/polysaccharide antigens. Among all IgG isotype deficiencies, a deficiency in IgG2 is the most common and is associated with recurring airway/respiratory infections in infants.
IgG3
IgG3 comprises around 5 to 10% of total IgG and plays a major role in the immune responses against protein or polypeptide antigens.
IgG4
Comprising usually less than 4% of total IgG, IgG4 does not bind to polysaccharides. Recent studies have shown that elevated serum levels of IgG4 are found in patients suffering from sclerosing pancreatitis, cholangitis and interstitial pneumonia caused by infiltrating IgG4 positive plasma cells. The precise role of IgG4 is still mostly unknown.
Functions of Immunoglobulin G (IgG)
- IgG is the main type of antibody found in blood and extracellular fluid allowing it to control infection of body tissues.
- IgG is the only class of immunoglobulin that can cross the placenta in humans, and it is largely responsible for protection of the newborn during the first months of life.
- IgG is the major immunoglobulin in blood, lymph fluid, cerebrospinal fluid and peritoneal fluid and a key player in the humoral immune response.
- The binding of the Fc portion of IgG to the receptor present on a phagocyte is a critical step in the opsonization. Phagocytosis of particles coated with IgG antibodies is a vital mechanism that cells use to cope with microorganisms.
- IgG is produced in a delayed response to an infection and can be retained in the body for a long time. The longevity in serum makes IgG most useful for passive immunization by transfer of this antibody. Detection of IgG usually indicates a prior infection or vaccination.
- Because of its relative abundance and excellent specificity toward antigens, IgG is the principle antibody used in immunological research and clinical diagnostics.
References
- https://www.thermofisher.com/np/en/home/life-science/antibodies/antibodies-learning-center/antibodies-resource-library/antibody-methods/immunoglobulin-igg-class.html
- https://www.genscript.com/IgG-antibody.html
- https://www.sciencedirect.com/topics/neuroscience/immunoglobulin-g
- https://www.bio-rad-antibodies.com/igg-immunoglobulin-g-antibodies.html
- https://en.wikipedia.org/wiki/Immunoglobulin_G
- http://mainebiotechnology.com/igg-function/

v informative
GOOD WORK DONE