- Immunoglobulin D (IgD) is a unique immunoglobulin with a low concentration in serum and the exact function of which is not known.
- IgD represents about 0.25% of the total serum immunoglobulins and has a relative molecular mass of 185 kDa while a half-life of 2.8 days, similar to that of IgE.
- However, it makes up about 1% of proteins in the plasma membranes of B-lymphocytes where it is usually co-expressed with another cell surface antibody, IgM. B cells thus can express both IgM and IgD which are specific for the same antigen.
- The co-expression is found on the surface of majority of B cells. IgD starts to be expressed when the B cell exits the bone marrow to populate peripheral lymphoid tissues. When a B cell reaches its mature state, it co-expresses both IgM and IgD.
- IgD is also produced in a secreted form that is found in very small amounts in blood serum.
Interesting Science Videos
Structure of IgD
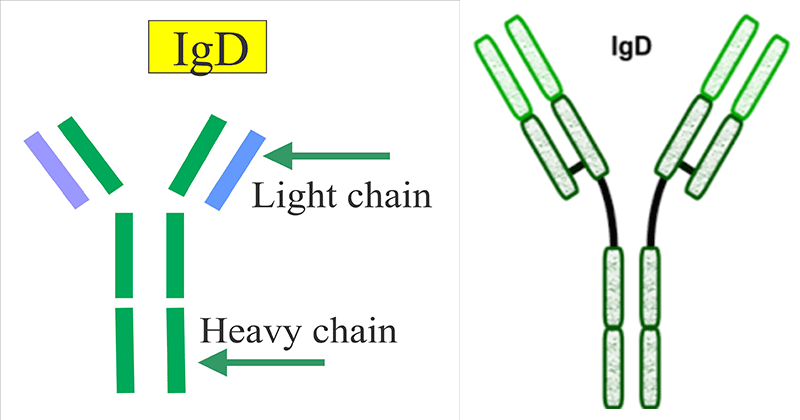
IgD is an immunoglobulin similar in structure to other immunoglobulin classes. It is composed of heavy and light polypeptide chains, has a sedimentation coefficient of approximately 7S, and can be fragmented into Fab and Fc fragments.
- Secreted IgD is a glycoprotein produced as a monomeric antibody with two identical heavy chains of the delta (δ) class, and two identical Ig light chains.
- The structure has two identical antigen binding areas consisting of both light and heavy chains and a valency of 2. Heavy and light chains have variable regions on their most N terminal ends.
- IgD on the surface of B cells has extra amino acids at C-terminal end for anchoring to the membrane. It also associates with the Ig-alpha and Ig-beta chains.
- Heavy and light chains are subdivided into variable and constant regions. In addition to the disulfide bonds linking the chains together, there are intrachain disulfide links that divide each chain into areas called domains.
- The light chains have two domains, one variable and one constant.
- The heavy chains have four domains, one variable and three constant -region domains.
- The IgD molecule has a long “hinge” region (between Fab and Fc) that appears to render the molecule very susceptible to proteolytic degradation with production of Fab and Fc fragments and also renders the molecule flexible, thus enhancing antigen binding.
Functions of IgD
While the function of IgM, IgG, IgA and IgE is relatively well known, the function of IgD has remained obscure since its discovery in 1965.
- This immunoglobulin functions primarily as an antigen receptor on B cells and is probably involved in regulating B cell function when it encounters antigen.
- When IgM and IgD expressed on a B cell interact with an antigen for which they are specific, the antigen is internalized, and processed and presented to helper T cells which trigger the B cells to proliferate and differentiate into plasma cells, thus initiating the development of a humoral immune response.
- Secreted IgD also exists and plays an elusive function in blood, mucosal secretions and on the surface of innate immune effector cells such as basophils. IgD apparently is also able to bind to basophils and mast cells and activate these cells to produce antimicrobial factors that are functional in respiratory immune defence in humans.
- It is also proposed that IgD may have some role in allergic reactions.
References
- Chen, K., & Cerutti, A. (2011). The Function and Regulation of Immunoglobulin D. Current Opinion in Immunology, 23(3), 345–352.
- Adrian O. Vladutiu.(2000). Immunoglobulin D: Properties, Measurement, and Clinical Relevance .Clin. Diagn. Lab. Immunol. 7(2): 131-140.
- http://www.microbiologybook.org/mobile/m.immuno-4.htm
- Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers.
- Owen, J. A., Punt, J., & Stranford, S. A. (2013). Kuby Immunology (7 ed.). New York: W.H. Freeman and Company.

DEAR Sir, If immunoglobulin D is used in abortion clinics to improve a woman’s immune system after an abortion, and to relieve reuses factor blood incompatibility, why cannot chemistry include a cure for this IgD immunoglobulin overdose so the human body can eliminate the drug through immune chemistry? mpennekamp@ivytech.edu