Interesting Science Videos
Structure of Human Cytomegalovirus
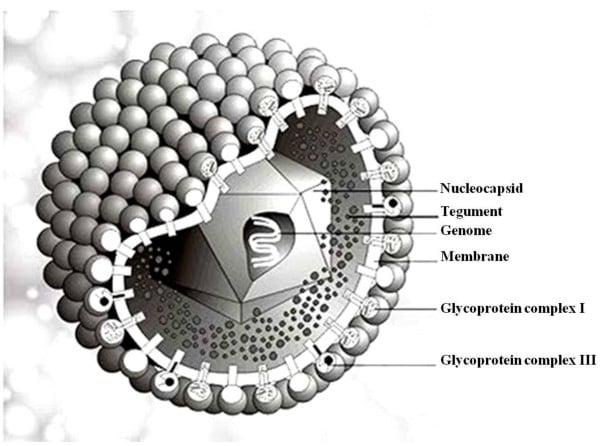
Figure: Structure of Human Cytomegalo Virus, Source: Dr. Marko Reschke, Marburg, Germany
- Cytomegalovirus is a member of the herpes virus family that falls under the beta herpes subfamily.
- CMV is spherical in shape with icosahedral symmetry.
- The icosahedral protein capsid with an average diameter of 100 nm consists of 162 hollow hexagonal and pentagonal capsomeres with an electron-dense core containing the double-stranded DNA genome with 230 kbp nucleotides together forming the nucleocapsid.
- The nucleocapsid is surrounded by an envelope which is lipoprotein in nature.
- The lipid part is derived from the nuclear membrane of the infected host cell.
- Projecting from the trilaminar lipid host-derived envelope are spikes of viral glycoproteins which bind to specific host receptor and mediate virus entry.
- gB is one of the major surface glycoproteins in CMV which influences virus binding to and entry into the cell and cell to cell transmission.
- gH is a second relatively abundant glycoprotein which is a target for complement independent neutralizing antibodies.
- In mature virus particles, outside the capsid is an amorphous proteinaceous layer, the tegument, surrounded by a lipid envelope derived from host cell membranes.
- At least 25 proteins are located in the tegument layer.
- The tegument consists of enzymes such as VP16 which is responsible for subverting cellular proteins and enzymes to involve in viral nucleic acid replication and VHS (Virion Host Shutoff) protein which shut off the host cell protein synthesis in the cytoplasm.
Genome of Human Cytomegalovirus

Figure: Genome of Human Cytomegalo Virus, Source: https://doi.org/10.3390/v4112448
- The CMV genome consists of approximately 230kb of linear double-stranded DNA.
- The genome is monopartite and contains terminal and internal reiterated sequences.
- The genome is split into a unique long (UL) and a unique short (US) region, both flanked by inverted sequences.
- It contains approximately 150 open reading frames (ORFs) that encode proteins.
- Out of these, it has been found that 41 are essential and 117 are nonessential for CMV replication.
Transmission and Epidemiology
- CMV infections occur worldwide and the prevalence of infection varies considerably.
- CMV is transmitted across the placenta within the birth canal and quite commonly in breast milk, in young children commonly through saliva.
- It can also be transmitted sexually showing presence in both semen and cervical secretions.
- Furthermore, it can also be transmitted via blood transfusion and during organ transplantation.
- Congenital CMV infection is the most common viral infection of human fetuses with 1% in the USA and 2-3% in developing countries.
Replication of Human Cytomegalovirus
- Attachment to host cells is mediated by the fusion of the viral glycoproteins to host receptors which further mediates endocytosis of the virus into the host cell.
- After entry and uncoating, the capsid is transported to the nuclear pore where the viral DNA is released into the nucleus.
- Tegument proteins regulate host cell responses and initiate the temporal cascade of the expression of viral I immediate-early (IE) genes, followed by delayed early (DE) genes, which initiate viral genome replication, and late (L) genes.
- Late gene expression initiates capsid assembly in the nucleus, followed by nuclear egress to the cytosol.
- Capsids associate with tegument proteins in the cytosol and are trafficked to the viral assembly complex (AC) that contains components of the endoplasmic reticulum (ER), Golgi apparatus and endosomal machinery.
- The capsids further acquire tegument and viral envelope by budding into intracellular vesicles at the assembly complex.
- Assembly of the virus in nuclear viral factories and budding through the inner lamella of the nuclear membrane by the insertion of herpes glycoproteins, throughout the Golgi and final release by exocytosis at the plasma membrane.
Image Source: MDPI Viruses
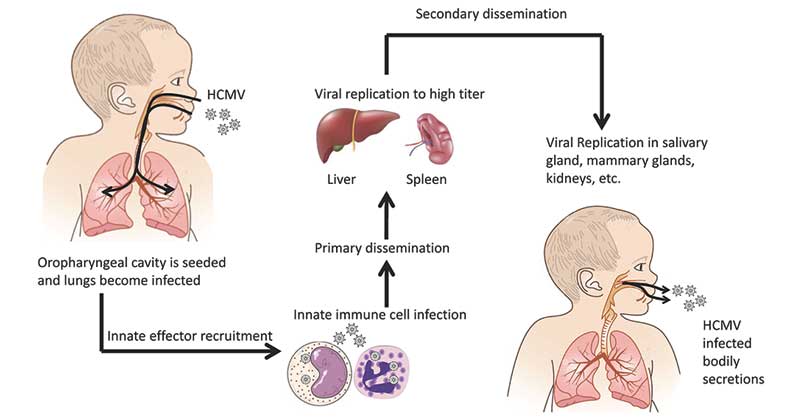
Pathogenesis of Human Cytomegalovirus
- HCMV infects and replicates in a wide variety of cells, including epithelial cells of the gland and mucosal tissue, smooth muscle cells, fibroblasts, macrophages, dendritic cells, hepatocytes, and vascular endothelial cells.
- This broad cell tropism facilitates systemic spread in the human body and inter-host spread.
- Primary replication typically starts with replication in the mucosal epithelium as a result of direct contact with infectious secretions from an infected individual.
- A systemic phase of infection disseminates virus in the host via leukocyte associated viremia that may last for several months.
- In addition, HCMV undergoes latency in myeloid cells of the bone marrow, presumably leading to a life-long infection with sporadic reactivation.
- HCMV infection is generally asymptomatic in healthy individuals. However, in immunocompromised individuals, such as organ transplant recipients or human immunodeficiency virus carriers, HCMV poses a life-threatening risk.
- HCMV is also recognized as the leading infectious cause of congenital neurological disease by transmission through the placenta from the mother to the child.
- CMV specific IgM antibodies are produced during the primary infection and persist for 3 or 4 months, but are not produced in recurrent infections in immunocompetent individuals.
- CMV IgG antibodies are produced at the time of primary infection and persist lifelong.
- With intrauterine infections, both IgM and IgG are produced by the fetus but the fetal IgG response can only be detected as the passively acquired IgG from the mother is catabolized.
- On the other hand, Cell-mediated immunity (CMI) is thought to play a key role in the suppression of CMI infection.
Clinical manifestations of Human Cytomegalovirus
- Acute CMV infection may mimic infectious mononucleosis caused by Epstein-Barr virus or liver infection by hepatitis A, B, or C.
- Mono-like symptoms may include fever, malaise, enlarged lymph nodes, sore throat, muscle aches, loss of appetite, enlarged liver or spleen, and fatigue.
- Hepatitis-like symptoms and signs may include appetite loss, yellow eyes, nausea, and diarrhea.
- In people with suppressed immune systems, CMV infection can attack different organs of the body and may cause blurred vision and blindness (CMV retinitis), lung infection (pneumonia), painful swallowing (esophagitis), diarrhea(colitis), inflammation of the liver (hepatitis), or inflammation of the brain (encephalitis), which may cause behavioral changes, seizures, or coma.
- Infants with CMV infection at birth (congenital CMV) have no symptoms at birth, however, up to 20% of those without symptoms at birth will go on to develop deafness.
- Only about 10% of infants with congenital CMV show signs and symptoms of the infection or develop complications.
- Signs and symptoms of CMV at birth may include deafness, yellow skin, and eyes (jaundice), skin rash, premature birth, low birth weight, pneumonia, enlarged liver and spleen, microcephaly, or seizures.
- The effects of congenital CMV infection include:
- CNS abnormalities – microcephaly, mental retardation, spasticity, epilepsy, periventricular calcification
- choroidoretinitis and optic atrophy
- sensorineural deafness
- hepatosplenomegaly and jaundice which is due to hepatitis
- pneumonitis
- myocarditis
- Thrombocytopenic purpura
- Hemolytic anemia
- Late sequelae – damage to the enamel forming organ of the teeth resulting in yellow discoloration of the teeth and brittleness.
Laboratory diagnosis of Human Cytomegalovirus
Specimen: urine, saline, tissue biopsy and other body fluids
- Virus isolation
- Human embryo lung fibroblasts are most commonly used.
- The specimen is inoculated into HEL cells and kept for 28 days with a blind passage at 14 days.
- CMV produces a typical focal cytopathic effect.
- Microscopy
- Virions in the urine of congenitally infected infants may be visualized by EM
- Cytopathology
- Intracellular inclusions surrounded by a clear halo may be demonstrated with various stains (Giemsa, Wright, hematoxylin-eosin, Papanicolaou).
- Cytomegalic inclusions can be recognized from biopsy material by the typical “owl’s eyes appearance “.
- Tissue immunofluorescence
- Infected lung and liver cells may be stained by specific anti-CMV antibodies.
- Immunohistochemistry
- Immunohistochemistry is performed primarily on tissue or body fluid samples.
- Slides are made from frozen sections of biopsy tissue samples (liver, lung) or by centrifuging cells onto a slide.
- Then monoclonal or polyclonal antibodies against early CMV antigens are applied and visualized by fluorescently labeled antibodies or enzyme-labeled secondary antibodies which are visualized by the change of color of the substrate.
- The stained slides are then examined by fluorescent or light microscopy.
- Antigen detection
- Antigenemia is defined as the detection of the CMV pp65 antigen in leukocytes.
- The pp65 assay is used to detect messenger matrix proteins on the CMV virus, with either immunofluorescence assay or messenger RNA amplification
- Serology
- Serological tests are useful for determining whether a patient has had CMV infection in the past, determined by the presence or absence of CMV IgG.
- The methods used for detection include complement fixation, enzyme-linked immunosorbent assay (ELISA), anti-complement immunofluorescence, radioimmunoassay, and indirect hemagglutination.
- The detection of IgM antibodies has been used as an indicator of acute or recent infection.
- The IgM capture assays are widely employed and are based on selective binding of IgM antibody to the solid phase.
- Molecular methods
- Polymerase chain reaction (PCR) is a widely available rapid and sensitive method of CMV detection based on the amplification of nucleic acids.
- PCR for CMV DNA can be either qualitative or quantitative, in which the amount of viral DNA in the respective sample is measured.
- The quantitative PCR (Real-Time PCR) allows for continuous monitoring of immunocompromised individuals to identify patients at risk for CMV disease for preemptive therapy and to determine response to treatment.
- Nucleic acid sequence-based amplification (NASBA) assay allows the specific nucleic assay sequence-based amplification of unspliced viral mRNAs (late pp67 mRNA expression) in a background of DNA using a specific isothermal technique of amplification.
Treatment
- The drugs ganciclovir and foscarnet have been licensed for use in life-threatening CMV infection in immunocompromised patients.
- Ganciclovir remains the mainstay of treatment in severe CMV disease other than retinitis.
- Foscarnet and cidofovir are used as second-line drugs.
- Acyclovir and valacyclovir have shown some benefits in the bone marrow and renal transplant patients.
Prevention and control
- Isolation of newborns with generalized cytomegalic inclusion disease from other newborns is advisable.
- Screening of transplant donors and recipients for CMV antibody.
- Administration of human IgG prepared from plasma pools has given discordant results in tests to decrease the incidence of viral infections in transplant recipients.
- Both live and recombinant vaccines are under development.
Sources
- 6% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3730495/
- 6% – https://www.medicinenet.com/cytomegalovirus_cmv/article.htm
- 3% – https://www.slideshare.net/abdullahabobakr7/cytomegalovirus-27430084
- 3% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4604749/
- 2% – https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/herpesviridae
- 2% – https://www.ijcmas.com/vol-4-11/M.%20Ram%20Mohan%20Rao%20and%20Syam%20D%20Gopal.pdf
- 2% – https://emedicine.medscape.com/article/215702-workup
- 2% – http://www.virology-online.com/viruses/CMV2.htm
- 2% – http://virology-online.com/viruses/CMV.htm
- 1% – https://www.youtube.com/watch?v=K-fdNrfbhUM
- 1% – https://www.virology-online.com/viruses/CMV.htm
- 1% – https://www.sciencedirect.com/science/article/pii/016609349090077S
- 1% – https://www.researchgate.net/publication/12293630_Viral_entry_into_the_nucleus
- 1% – https://www.pnas.org/content/pnas/100/24/14223.full.pdf
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385066/
- 1% – https://viralzone.expasy.org/complete_by_species/18.html
- 1% – https://viralzone.expasy.org/by_species/180
- 1% – https://quizlet.com/30877982/micro-mini-6-flash-cards/
- 1% – https://quizlet.com/207698031/chapter-9-bio-02-flash-cards/
- 1% – https://academic.oup.com/tropej/article/61/2/79/1728980
- 1% – http://www.signavitae.com/2015/06/congenital-cytomegalovirus-infection-case-report/
- <1% – https://www.sciencedirect.com/topics/chemistry/double-stranded-dna
- <1% – https://www.researchgate.net/publication/51046937_Cytomegalovirus_CMV
- <1% – https://www.researchgate.net/publication/265514663_The_pathogenesis_of_human_cytomegalovirus
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3144558/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2885759/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2415745/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1563938/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC112471/
- <1% – https://www.nature.com/articles/6364293
- <1% – https://www.medscape.com/answers/215702-99981/which-medications-are-used-in-the-management-of-cytomegalovirus-cmv-disease
- <1% – https://www.mdpi.com/1422-0067/20/15/3626/pdf-vor
- <1% – https://www.klinikum.uni-heidelberg.de/zentrum-fuer-infektiologie/virologie/forschung/forschungsgruppen/kraeusslich/
- <1% – https://quizlet.com/49419439/chapter-6-flash-cards/
- <1% – https://labtestsonline.org/articles/anatomic-pathology
- <1% – https://laboratoryinfo.com/elisa/
- <1% – https://ijponline.biomedcentral.com/articles/10.1186/s13052-017-0358-8
- <1% – https://aem.asm.org/content/71/11/7113
- <1% – http://www.plantcell.org/content/11/4/643
- <1% – http://www.authorstream.com/Presentation/doctorrao-2175697-cytomegalovirus/
- <1% – http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.1962.tb08674.x/abstract
- <1% – http://europepmc.org/articles/PMC138598