Structure of Human Astrovirus
- Human astrovirus falls under Astroviridae and genus Mamastrovirus.
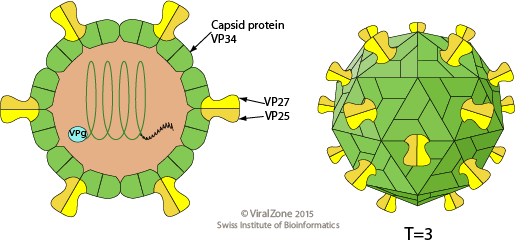
- The particles are round in shape, 28 nm in diameter with a smooth margin and display a five or six pointed star motif on their surface.The term astrovirus (Greek: astron- star) was coined to refer this feature.
- They are non-enveloped, spherical, capsid of about 35 nm with icosahedral symmetry and comprises single stranded RNA with positive polarity.
- Surface projections are small and surface appears rough, spikes protruding from the 30 vertices.
Source: Viral Zone, Swiss Institute of Bioinformatics
- The capsid precursor protein (180 copies per particle) undergoes C-terminal cleavages by host caspases during virus maturation.
- Infectious particles are generated by further cleavages of VP70 by extracellular proteases resulting in three structural proteins.
- VP34 is derived from the highly conserved N-terminal region of the polyprotein and builds up the capsid shell, while VP27 and VP25 are both derived from the variable C-terminal domain with a different N terminus and form the dimeric spikes.
- They have a buoyant density of between 1.35 and 1.37 g/ml in CsCl.
- There are at least 8 distinct serotypes (HAstV 1–8) of human astrovirus, defined both antigenically and by genetic sequence differences.
Genome of Human Astrovirus
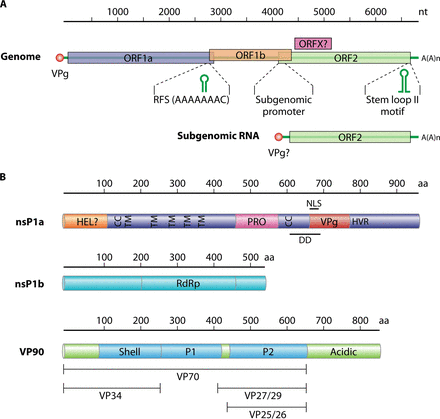
- The genome is monopartite, linear ssRNA(+) genome of 6.8-7kb in size.
- The 5′-terminus is linked to a VPg protein and the 3′-terminus has a poly(A) tract.
- The virion RNA is infectious and serves as both the genome and viral messenger RNA.
Source: doi: 10.1128/CMR.00013-14
- The genome contains three overlapping open reading frames (ORF1a, ORF1b, and ORF2).
- The nonstructural proteins are translated from the genomic RNA as two large polyproteins, nsP1a and nsP1a/1b, through a translational ribosomal frameshifting
- ORF1a and ORF1b encode the viral protease and polymerase respectively.
- ORF2 is expressed from a subgenomic RNA and encodes the VP90 capsid precursor protein.
Transmission and Epidemiology of Human Astrovirus
- Astroviruses are transmitted from person-to-person by the faecal–oral route or through contaminated food or water.
- The environmental transmission of HAstV is supported by the stability of the virus in drinking water, freshwater, and marine water
- Astrovirusis worldwide in distribution and tends to infect mainly children in the 1- to 3-year age group.
- Astroviruses have also been associated with foodborne outbreaks of gastroenteritis in Japanese adults and school children.
- Astroviruses appear to account for 4 to 8.6% of all young children with diarrhea, depending on geographic location.
- Infections occur primarily in winter time in temperate climates, as has been reported for rotavirus.
- Studies have shown that astroviruses can cause sporadic outbreaks of diarrhea in elderly patients and that it is significantly associated with diarrheal illness in immunocompromised AIDS patients, solid organ transplant recipients, and bone marrow transplant recipients.
Replication of Human Astrovirus
Source: Schultz-Cherry, S. Astrovirus Research: Essential Ideas, Everyday Impacts, Future Directions; Springer: New York, NY, USA, 2013; pp. XIV, 185.
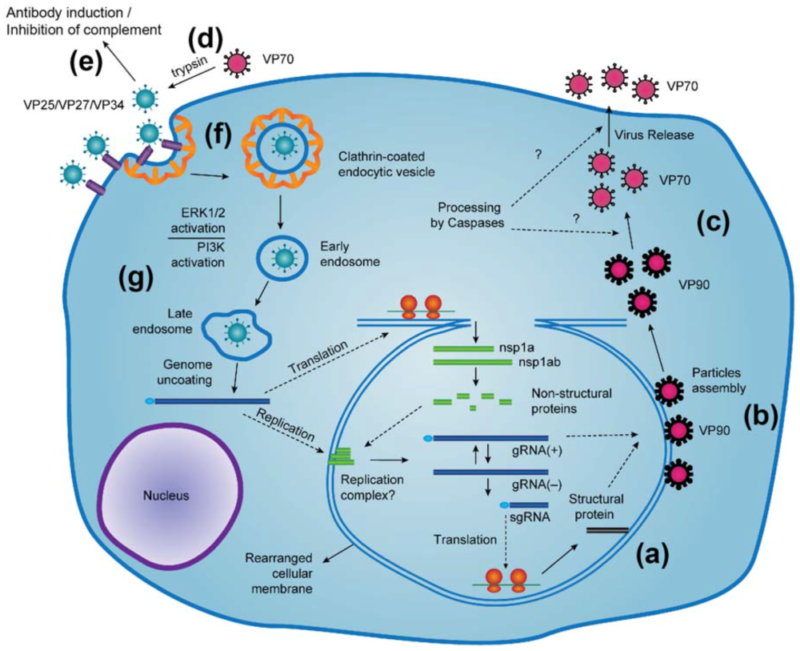
- Attachement to host receptors probably mediates endocytosis of the virus into the host cell.
- Uncoating, and release of the viral genomic RNA into the cytoplasm.
- The genome is then translated, giving rise to nsP1a1b and nsP1a polyproteins, which are then cleaved by the viral serine protease (in nsP1a) as well as some cellular proteases, resulting in the individual nonstructural protein.
- Proteolytic processing of polyproteins nsP1a1b (around 160 kDa) produces the nsP1b protein (RdRp) (around 57 kDa) and the nsP1a protein (around 102 kDa), which is subsequently cleaved to yield several mature products.
- Replication occurs in viral factories made of membrane vesicles derived from the ER.
- A full-length negative-sense genomic RNA would be synthesized and used as a template for the production of both positive-sense genomic RNA and positive-sense subgenomic RNA.
- Subgenomic RNA translation gives rise to the capsid protein precursor VP90.
- VP90 polyprotein initially assembles into immature virions in association with intracellular membranes through its C terminus and that several cellular caspases further cleave these VP90 polyproteins close to their C termini once they have dissociated from membranes, resulting in viral capsids composed of 70-kDa polyproteins (VP70).
- Finally, VP70-containing viral particles would be released from cells and proteolytically processed by trypsin to enhance infectivity.
- Virus release by cell lysis and maturation of the capsid by proteolytic cleavages.
Pathogenesis of Human Astrovirus
- Spread of the virus may occur via the fecal-oral route from person-to-person contact or through contaminated food or water.
- Astrovirus pathogenesis has not been well studied in humans.
- Viral particles have been visualized by EM in intestinal epithelial cells and in epithelial cells located in the lower part of the villi, suggesting that the intestine is the site of replication but systemic spread has been described in immunocompromised children.
- Astroviruses cause gastroenteritis by causing destruction of the intestinal epithelium, leading to the inhibition of usual absorption mechanism, loss of secretory functions, and decrease in epithelial permeability in the intestines.
- Although mucosal IgA levels in the gastrointestinal tract have been shown to be major immune effector products against gastrointestinal viral infections and IgAs are induced upon AstV infection, it is yet to be elucidated whether they are required for protection.
- Intestinal biopsy specimens from healthy adults in an organ culture system with inactivated HAstVs demonstrated the presence of HAstV-specific CD4+and CD8+ T cells residing in the normal tissue.
- Thus, it shows that both humoral and cellular adaptive immune responses are involved in protecting normal healthy adults from reinfections.
- Very high levels of virus (up to 1013 genome copies/g) can be present in stools, and virus excretion can be detected for up to two weeks after symptoms have cleared.
Clinical manifestations of Human Astrovirus
- Astrovirusgastroenteritis, seen principally in young children, is usually clinically mild.
- A mean incubation period of 4 to 5 days is followed by a watery diarrhea lasting from one to four days or more along with abdominal discomfort, vomiting, fever, and anorexia.
- Vomiting is less common than in rotavirus or calicivirus infection, and the disease is usually milder.
- Children may shed virus 1–2 days prior to illness and for 4–5 days following illness.
- Children with poor nutritional status may develop more severe disease or chronic diarrhea.
Diagnosis of Human Astrovirus
- HAstVs are routinely detected by direct transmission electron microscopy (EM) in negatively stained stool samples.
- Although cell culture propagation of some classic HAstVs is possible with the aid of trypsin, virus isolation in cell culture is not applicable for astrovirus diagnosis in fecal specimens.
- Enzyme immunoassay using monoclonal antibody to the group antigen to capture virus.
- A latex agglutination test specific for HAstV-1 serotype has been reported.
- ELISA and immunofluorecence based methods to detect serum antibody.
- RT-PCR has been used for genotyping of human astroviruses.
Treatment of Human Astrovirus
- No antiviral therapy
- Supportive treatments include oral or intravenous fluid replacement to avoid dehydration.
Prevention and control of Human Astrovirus
- No vaccine available.
- The control of the transmission routes includes virus detection and inactivation in water and food and disinfection of contaminated fomites.
