Interesting Science Videos
Structure of Hepatitis E Virus

Image: Schematic illustration of non-enveloped and quasi-enveloped HEV particles as well as enveloped virus. The putative model of quasi-enveloped HEV virion includes ORF3 product in its envelope as the existence of pORF3 has been confirmed by capturing quasi-enveloped HEV virion with anti-pORF3 antibodies and further supported by prediction of a putative transmembrane region in the N-terminal of pORF3.
Source: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00266/full
- HEV is classified in the Calciviridae family because of its structural similarity to other calciviruses; however, it is now the sole member of the Hepeviridae family.
- The virus is non enveloped (naked) with icosahedral symmetry measuring 27-30 nm diameter.
- The genome is single stranded RNA genome with positive polarity and measure about 7.2 kb in length.
Genome of Hepatitis E Virus
- The HEV genome is a single-stranded, positive-sense RNA molecule and 7.2 kb in size.

- Genomic RNA is polyadenylated and contains 3 ORFs.
- ORF1 encodes the nonstructural proteins, ORF2 encodes the capsid protein, and ORF3 encodes a small multifunctional protein.
- The ORF2 and ORF3 proteins are translated from a single, bicistronic mRNA.
- Located near the 5′-end, ORF1 encodes a non-structural polyprotein with multiple functional domains, including those for methyltransferase, protease, helicase, and polymerase.
- The viral capsid protein (CP) is encoded by ORF2 near the 3′-end.
- ORF3, which partially overlaps with the other 2 ORFs, codes for an immunogenic protein of unknown function.
- ORF3 encodes a 113 or 114 aa phosphoprotein, depending upon the genotype.
- The ORF2 capsid protein, HEV-CP, contains a total of 660 amino acid residues.
- At the HEV-CP N terminus is a signal peptide followed by an arginine-rich domain that potentially play a role in viral RNA encapsidation during assembly.
- HEV-CP is a key antigen that stimulates the host immune response, and 6 antigenic domains have been identified.
- One neutralization site has been mapped to the polypeptide region between amino acids 452 and 617.
Epidemiology and Transmission of Hepatitis E Virus
- The HEV infection was first reported from the Indian subcontinent and subsequently from other parts of Asia, the Middle East, Central and South America, Africa, Central Europe and Russia.
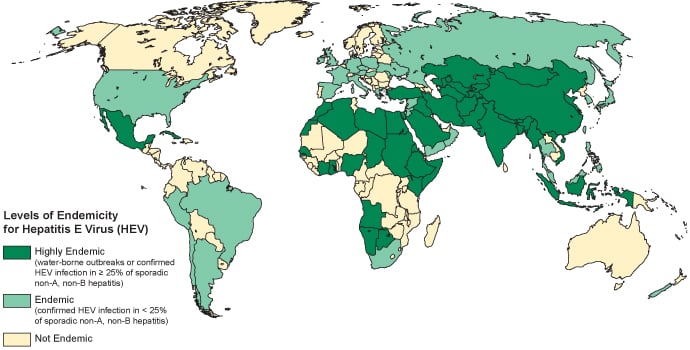
- People travelling to countries with high prevalence are therefore at risk of acquiring infection during their travel.
- Adult populations in endemic areas are generally susceptible and there is a high infection rate in epidemics.
- The hepatitis E virus is transmitted mainly through the faecal-oral route due to faecal contamination of drinking water or via Ingestion of undercooked meat or meat products derived from infected animals.
- Other transmission route includes: vertical transmission from a pregnant woman to her fetus and transfusion of infected blood products.
Replication of Hepatitis E Virus
- The mechanisms underlying HEV replication are poorly understood.
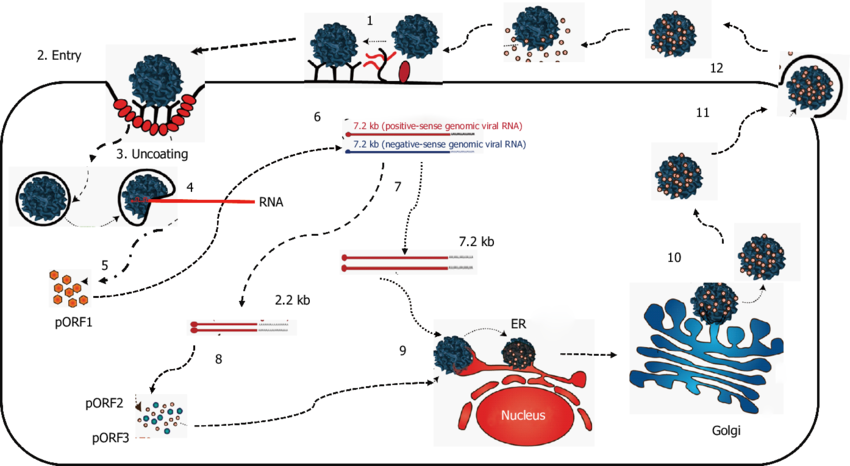
Figure: Proposed replication of hepatitis E virus. Source: Doi: 10.1111/jvh.12445.
- The HEV capsid protein is believed to bind to a cellular receptor to initiate viral entry and replication.
- ORF2 peptide-binding experiments suggested that the C-terminal region of ORF2 may mediate virus entry by binding to heat shock cognate protein 70 (HSC70) on the cell surface.
- Additionally, HSPGs have been identified as attachment receptors that are located on the cell surface.
- After virus entry into permissive cells, the HEV genomic RNA is uncoated by unknown mechanisms.
- After uncoating, virion releases the positive-sense genomic RNA into the cytoplasm of the cell.
- The positive-sense genomic viral RNA serves as the template to translate the ORF1 nonstructural polyprotein in the cytoplasm.
- The viral RdRp synthesizes an intermediate, replicative negative-sense RNA from the positive-sense genomic RNA that serves as the template for the production of positive-sense, progeny viral genomes.
- The ORF2 and ORF3 proteins are translated from the subgenomic, positive-stranded RNA, and the ORF2 capsid protein packages the genomic viral RNA and assembles new virions.
- The nascent virions are transported to the cell membrane.
- The ORF3 protein facilitates the trafficking of the virion, and the nascent virions are released from the infected cells by lysis.
Pathogenesis of Hepatitis E Virus
- The pathogenesis of hepatitis E is poorly understood.

- Since HEV is presumably transmitted by the fecal-oral route, it is unclear how the virus reaches the liver.
- There is an extra-hepatic site of virus replication.
- The virus could replicate in the intestinal tract before reaching the liver.
- Negative strands of HEV RNA, indicating virus replication, have been detected in the small intestine, lymph nodes, colon, and liver of pigs, indicating extra-hepatic HEV replication.
- HEV then replicates in the cytoplasm of hepatocytes and is released into both blood and bile.
- The liver damage induced by HEV infection may be immune-mediated by cytotoxic T cells and natural killer (NK) cells since HEV is not cytopathic.
- The virus is shed in the stool.
- A serological anti-HEV response is generally detected in patients at the time of onset of illness.
- Anti-HEV IgMs are detected in the early phase of clinical illness, and can persist for several months.
- Anti-HEV IgG appears shortly after the IgM response and can last several years.
- Cross protection is possible due to the existence of only one serotype.
Pathogenesis of fulminant Hepatitis
- The reasons why a hepatitis E infection becomes fulminant are still obscure.
- Stimulating both Th1 and Th2 type immune responses could play a role in liver failure.
- Host factors rather than virus genotype, variants, or specific aminoacids substitutions are responsible for the development of fulminant hepatitis.
- In fulminant hepatitis, there is higher anti-HEV IgM and IgG titers, along with higher concentrations of IFN-γ, TNF-α, IL-2, and IL-10.
- CD4+ T cells are more frequent in the liver and CD8+ T cells have been shown to infiltrate the liver of patients with fulminant hepatitis E.
- Thus, cytotoxic CD8+ T cells could be particularly important in the pathogenesis of fulminant hepatitis.
- Women with acute liver failure (ALF) presents a reduced expression of toll-like receptor (TLR) 3/TLR7/TLR9.
- Impaired monocyte-macrophage function in pregnant women with ALF could contribute to an inadequate innate immune response, and hence to the development and severity of ALF.
- High concentrations of cytokines (TNF-α, IL-6, IFN-γ and TGF-β1) may also be associated with an adverse pregnancy outcome.
- The concentrations of estrogen, progesterone, and β-human chorionic gonadotrophin in HEV-positive pregnant FHF (Fulminant Hepatic Failure) women are higher than in HEV-negative pregnant FHF women.
- An in vitro study have shown that serum from pregnant women, especially those in the third trimester, enhanced the replication of HEV by inhibiting estrogen receptor and type I IFN expression.
Clinical Manifestations of Hepatitis E Virus
- Most HEV infections have a clinically silent course.

- In symptomatic cases, the incubation period ranges from 2 to 8 weeks, with a mean of 40 days.
- Initial symptoms of acute hepatitis E are typically unspecific and include flu-like myalgia, arthralgia, weakness, and vomiting.
- Some patients have jaundice, itching, uncolored stools, and darkened urine, accompanied by increased levels of liver transaminases, bilirubin, alkaline phosphatase, and γ-glutamyltransferase.
- HEV infection can lead to more severe, acute liver disease in pregnant women or patients with underlying chronic liver diseases and sometimes progress to fulminant hepatic failure.
- Infection in organ transplant recipients: Chronic HEV infection has been described in liver and kidney transplant recipients,that lead to persistent increases in levels of alanine aminotransferase, significant histological activity, and fibrosis.
- Patients with HIV Infection: Human immunodeficiency viruses (HIV)–infected individuals more frequently have positive results from tests for anti-HEV than individuals without HIV infection.
- Extrahepatic Manifestations: Muscular weakness and a pyramidal syndrome in a kidney transplant recipient with persistent HEV infection.
- In addition, neurological disorders, including polyradiculopathy, Guillain–Barré syndrome, bilateral brachial neuritis, encephalitis, or proximal myopathy have been reported in patients with acute and chronic HEV infections.
Laboratory Diagnosis of Hepatitis E Virus
- Specimens: Blood, serum, stool
- Definitive diagnosis of hepatitis E infection is usually based on the detection of specific IgM and IgG antibodies to the virus in a person’s blood.
- Additional tests include reverse transcriptase polymerase chain reaction (RT-PCR) to detect the hepatitis E virus RNA in blood and/or stool.
Treatment of Hepatitis E Virus
- There is no specific treatment capable of altering the course of acute hepatitis E.
- The disease is usually self-limiting.
- Hospitalization is required for people with fulminant hepatitis, and should also be considered for symptomatic pregnant women.
- Immunosuppressed people with chronic hepatitis E benefit from specific treatment using ribavirin, an antiviral drug.
- In some specific situations, interferon has also been used successfully.
Prevention and Control of Hepatitis E Virus
- At the population level, transmission of HEV and hepatitis E disease can be reduced by:
- Maintaining quality standards for public water supplies.
- Establishing proper disposal systems for human feces.
- On an individual level, infection risk can be reduced by:
- Maintaining hygienic practices such as hand-washing with safe water, particularly before handling food.
- Avoiding consumption of water and/or ice of unknown purity, and adhering to WHO safe food practices.
- “HEV is preventable by vaccination. HEV239 (Hecolin) is a recombinant HEV vaccine against genotype 1 and 4 that has shown to have more than 95% protection against the virus and to be safe in pregnancy. This vaccine is now available in China”.

The HEV viral structure you have shown is incorrect. It is in fact of HIV. HEV doesn’t have RT (Reverse transcriptase) neither does it have most of the proteins marked in the figure. Please update ASAP as your site is one of the first ones to be suggested when HEV structure is googled. It is entirely misleading. It won’t affect researchers as they can recognize the HIV particle anywhere but will surely affect students still at the beginning of their journeys. These are the ones most likely to refer to your notes. And as there are some advertisements here, I guess you are earning from this site so it’s your obligation to at least provide the right information.
Thank you so much for your correction.
I have removed the wrong figure and updated with the correct one.