Interesting Science Videos
Structure of Hepatitis C virus
- Hepatitis C virus (HCV) is a RNA virus belonging to the family Flaviviridae, genus Hepacivirus.
- The HCV virion is 55-65 nm in diameter containing in it a 9.6 kb positive sense single-stranded RNA genome composed of a long open reading frame (ORF) flanked by untranslated regions (UTR’s) at both the ends.
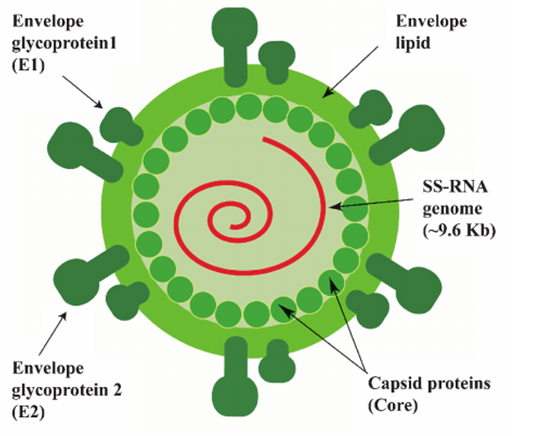
- The genome of HCV is thought to encode at least ten proteins, of which three are structural (core, envelope glycoproteins E1, and E2) and six nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B).
- HCV also expresses p7, a membrane-associated ion channel that may function during virus assembly or infection.
Genome of Hepatitis C Virus
- The HCV genomic RNA contains three distinct regions
- a 5′UTR or non-coding region
- a long open reading frame (ORF) of more than 9000 nucleotides (nt)
- a short 3′UTR.
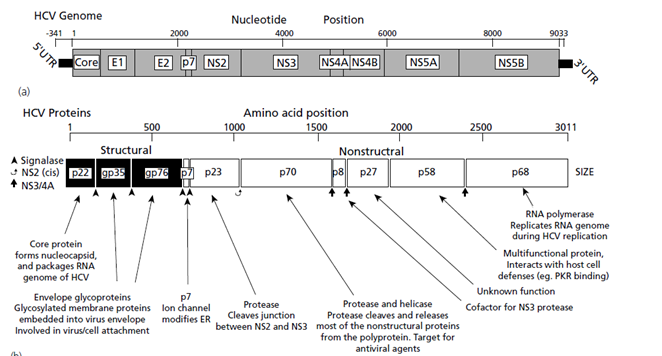
- The HCV 5’UTR contains 341 nucleotides located upstream of the ORF translation initiation codon.
- The 5′ UTR has a ribosome binding site or internal ribosome entry site (IRES) that initiates the translation of a very long protein
- The long ORF encodes a polyprotein of approximately 3000 amino acids, which will be further processed by host and viral proteases.
- The 3’UTR is principally involved in minus-strand priming during HCV replication.
- The 5’UTR is the most conserved region in the genome while the regions encoding envelope proteins (E1, E2) are the most variable ones.
- The long ORF encodes a polyprotein which is processed co- and posttranslationally by host and viral proteases into at least 10 different proteins, which are arranged in the order of NH2-C-E1-E2-p7-NS2-NS3-NS4A-NS4B-NS5A-NS5B-COOH.
- Host signal peptidase is required for the cleavages at C-E1, E1-E2, E2-p7, and p7-NS2 junctions.
- Two viral proteases are involved in the processing of HCV nonstructural proteins: NS2, a zinc-dependent metalloprotease that cleaves between NS2 and NS3; and NS3/4A, a serine protease that cleaves between the NS3-NS4A, NS4A-NS4B, NS4B-NS5A, and NS5A-NS5B junctions .
Epidemiology of Hepatitis C Virus
- Hepatitis C is a disease with a worldwide distribution, although the prevalence varies broadly among geographic areas.
- WHO estimates that about 170 million people, or 3 percent of the world’s population, are infected with HCV.
- The most affected regions are WHO Eastern Mediterranean and European Regions, with the prevalence of 2.3% and 1.5% respectively.
- The estimated global prevalence of HCV infection is 3% which translates to over 180 million people worldwide.
- High seroprevalence is observed in Asian and African countries, whereas the developed world including North America, Northern and Western Europe, and Australia have a low prevalence.
- In developing countries, the seroprevalence of HCV displays a high range of variability, ranging from 0.9% in India to higher prevalence from 2.1-6.5% in many countries.
- Egypt has a reported seroprevalence of about 22% and is the highest in the world.
- Substantial regional differences exist in the distribution of HCV genotypes in the world.
Transmission of Hepatitis C Virus
- HCV is transmitted from one person to another principally by parenteral route.
- The major routes of transmission are:
- Injection drug use
- blood transfusion
- unsafe therapeutic injections.
- Other routes of transmissions include
- healthcare related procedures (occupational exposures like needle stick injuries)
- tattooing
- perinatal transmission
- sexual transmission
Replication of Hepatitis C Virus
- The HCV lifecycle begins with the attachment of a virion to its specific receptors on hepatocytes: the high-density lipoprotein receptor , scavenger receptor class B type I, tetraspanin CD81, tight junction protein claudin-1, and occludin are the known cellular receptors initiating the attachment step of HCV infection.
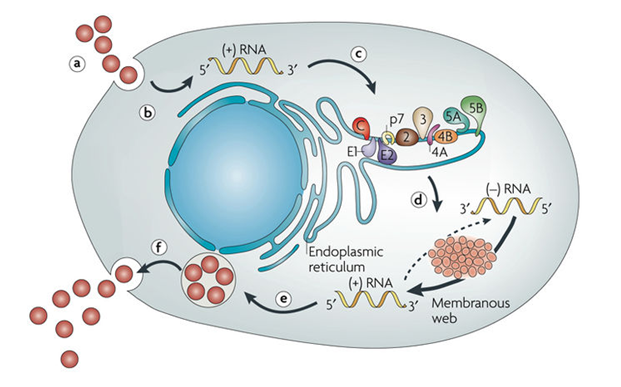
- Viral internalisation is through endocytosis of the bound virion in a clathrin-coated endosome, and fusion of viral and endosomal membranes and that the nucleocapsid is released into the cytoplasm.
- The virus is then uncoating to free its genomic RNA, and the HCV genomic RNA is used both for polyprotein translation and replication in the cytoplasm.
- Replication of HCV takes place in membranous webs associated with the NS4B protein and cellular endoplasmic reticulum (ER).
- HCV replication is catalyzed by the NS5B protein.
- NS4B initiates the formation of replication complex that supports HCV replication.
- The viral RNA-dependent RNA polymerase, first copies the positive sense RNA into a negative-sense RNA intermediate and then copies the negative-sense RNA into more positive sense RNA.
- The core protein, however, remains within the cytoplasm after cleavage from E1, E1 and E2 are embedded in the ER membrane, and their extracellular domains are glycosylated.
- Nascent genomes can then be translated, further replicated or packaged within new virus particles.
- New virus particles are thought to bud into the secretory pathway and are released at the cell surface.
Pathogenesis of Hepatitis C Virus
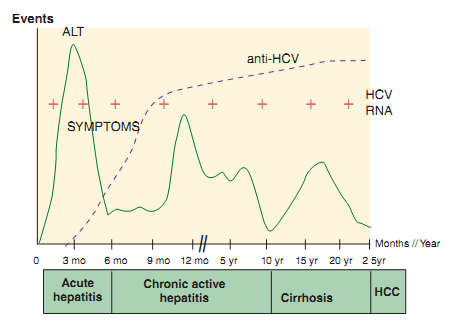
Figure: Clinical and serologic events associated with Hepatitis C virus (HCV) infection
- In most instances there is a slowly progressive asymptomatic hepatitis, with persistent viraemia.
- Those with chronic active disease are liable to proceed to cirrhosis, but this process is slow, taking as long as 20 years.
- Periodic exacerbations are marked by rises in alanine aminotransferase values.
- Although individuals vary in both specificity and timing of anti-HCV seroconversion, HCV-specific antibodies are generally detectable 7 to 31 weeks after infection.
- The humoral immune response is usually multispecific and targeted against epitopes within the HCV core, envelope, NS3, and NS4 proteins.
- Specifically, the triad of steatosis, bile duct damage, and lymphoid follicles within the portal tracts is characteristic for hepatitis C.
- Steatosis manifests as large droplets of fat vacuoles in the cytoplasm of hepatocytes.
- Finally, eosinophilic material in the cytoplasm of periportal hepatocytes is found in about 20% of chronic cases of HCV hepatitis.
- The level of necroinflammatory activity, fibrosis, and cirrhosis can vary widely and is not necessarily associated with an increased serum alanine aminotransferase activity.
- Disease progression to liver cirrhosis is promoted by various cofactors, such as co-infection with other hepatotropic viruses (e.g., HBV and alcohol intake ).
- Primary hepatocellular carcinoma (HCC) is a frequent complication of longstanding chronic active hepatitis.
- Destruction of infected hepatocytes may occur in several ways: HCV-infected hepatocytes can be killed by HCV-specific CTL clones by means of Fas ligand, TNF-α, or perforin-based mechanisms.
- One hypothesis is that the HCV-specific immune response is too weak to clear HCV from all infected hepatocytes once a persistent infection is established.
- Hypothetically, this may be due either to an insufficient induction of the primary immune response during acute infection or to the inability to maintain the T-cell response at high levels during chronic hepatitis C.
Clinical manifestations of Hepatitis C Virus
- Acute hepatitis
- The incubation period for hepatitis C averages about 7 weeks, with a range of 2 to 26 weeks, after exposure.
- Those who are acutely symptomatic may exhibit fever, fatigue, decreased appetite, nausea, vomiting, abdominal pain, dark urine, grey-coloured faeces, joint pain and jaundice (yellowing of skin and the whites of the eyes).
- Symptoms of malaise, nausea, and right upper quadrant pain followed by dark urine and jaundice appear in only about one third of patients.
- Chronic hepatitis
- Patients with chronic HCV infection may experience increased fatigue as their only symptom.
- Others may have overt symptomatic liver disease with anorexia, nausea, right upper quadrant pain, dark urine, and pruritus.
- ALT levels frequently fluctuate over time and may be normal or significantly elevated in the same patient measured at different times, whereas others have persistently normal or persistently elevated ALT levels.
- Hepatitis C patients presenting with significant chronic liver disease develop serious sequelae.
- Over a period of time, cirrhosis of liver may be seen.
- Hepatocellular carcinoma
- Chronic infection with either virus results in chronic inflammation, necrosis, regeneration, and cirrhosis, which are all related to increased risk for oncogenesis.
Lab Diagnosis of Hepatitis C Virus
- Antibody detection
- Anti-HCV is typically identified by using enzyme-linked immunosorbent assay (ELISA).
- Three generations of ELISAs have been developed
- First generation assays, which incorporated the recombinant c100-3 epitope from the NS4 region
- Second generation assays which additionally incorporated epitopes c22-3 and c33c from the HCV core and NS3 regions, respectively.
- Third generation assays contained reconfigured core and NS3 antigens and in addition a newly incorporated antigen from the NS5 region.
- The antigens utilized in the fourth generation anti-HCV assay are derived from the core (two different epitope clusters), NS3, NS4A, NS4B, as well as the NS5A regions.
- Recombinant immunoblot assays (RIBA) can be used to confirm the presence of anti-HCV antibodies.
- HCV core antigen detection
- Detected by ELISA or CLIA (chemiluminescence immunoassay).
- Detection of viral RNA
- Detection of HCV RNA by PCR and nucleic acid amplification tests (NAT), such as Transcript-Mediated Amplification (TMA)
Qualitative HCV RNA detection
- These assays commonly utilize conventional RT-PCR or transcription-mediated amplification (TMA).
Quantitative NAT
- Commonly available formats include quantitative RT-PCR (qRT-PCR) and branched deoxyribonucleic acid (bDNA) technology.
Treatment of Hepatitis C
- Interferon treatment
- Combination antiviral therapy with interferon-α and ribavirin
Vaccines of Hepatitis C
- HCV is highly heterogeneous, and effective vaccines may need to be multivalent in order to be protective against multiple serotypes.
Prevention and control of Hepatitis C
- Screening of blood donors and screening for presence of HCV before transfusion of blood.
- The use of sterile needles for medical and dental procedures, tattooing, and other percutaneous exposures.

Very useful information for new candidate. Thanks.
thank u so much sir
please sir help me with a concise seminar report on HEPATITIS C
very much informative and helpful for study
thank you so much sir
Useful Information for Students and General Public
Thank you so much.
Compliment of the season. Kindly send your e mail for discussion on collaboration on research. I’m a Medical Microbiologists and Ass. PROF. at Caleb University Lagos Nigeria.
Thank you
Thank you sir, I will email you for research collaboration. My email is me@sagararyal.com
Thanks for information if any Research work plz involve me I am ready to do hard work Dr raghavendra here assistant professor phd medical microbiology