Interesting Science Videos
Structure of Hepatitis A Virus
- HAV (Hepatitis A Virus) is a distinct member of the picornavirus family, assigned to genus hepatovirus.
- HAV is a 27 nm to 32 nm spherical particle with icosahedral symmetry containing a linear single-stranded RNA genome with a size of 7.5 kb and non-enveloped.
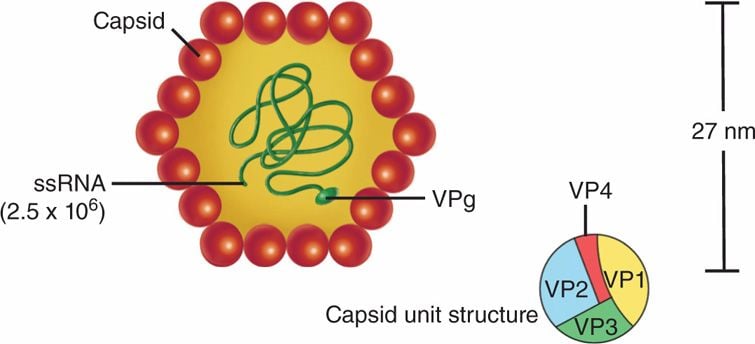
- Hepatitis A virions have a primary buoyant density of 1.32 to 1.34 g/cc in CsCl and a sedimentation coefficient of 156S to 160S in neutral sucrose solutions.
Genome of Hepatitis A Virus
- HAV can be divided into three parts
- a 5′ noncoding region (NCR) that comprises approximately 10% of the genome, is uncapped, and is covalently linked at the 5′ terminus to viral protein VPg
- a single open reading frame that appears to encode all of the viral proteins, with regions designated as P1 for capsid proteins and P2 and P3 for nonstructural proteins
- a short 3′ NCR terminating in a polyA tail.
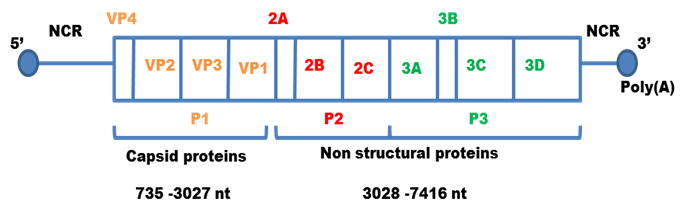
- The regions P1 contains four segments for structural proteins which make up the capsid protein; 1A-VP4, 1B- VP2, 1C-VP3, 1D-VP1.
- P2 comprises of three non structural proteins; 2A, 2B, 2C which play a role in viral replication.
- P3 makes up four non structural proteins
- 3A- anchors the replication complex to cell membrane
- 3B- it is VPg protein
- 3C- it is cysteine protease that cleaves the protein from polypeptides
- 3D- it is RNA dependent RNA Polymerase.
Epidemiology of Hepatitis A Virus
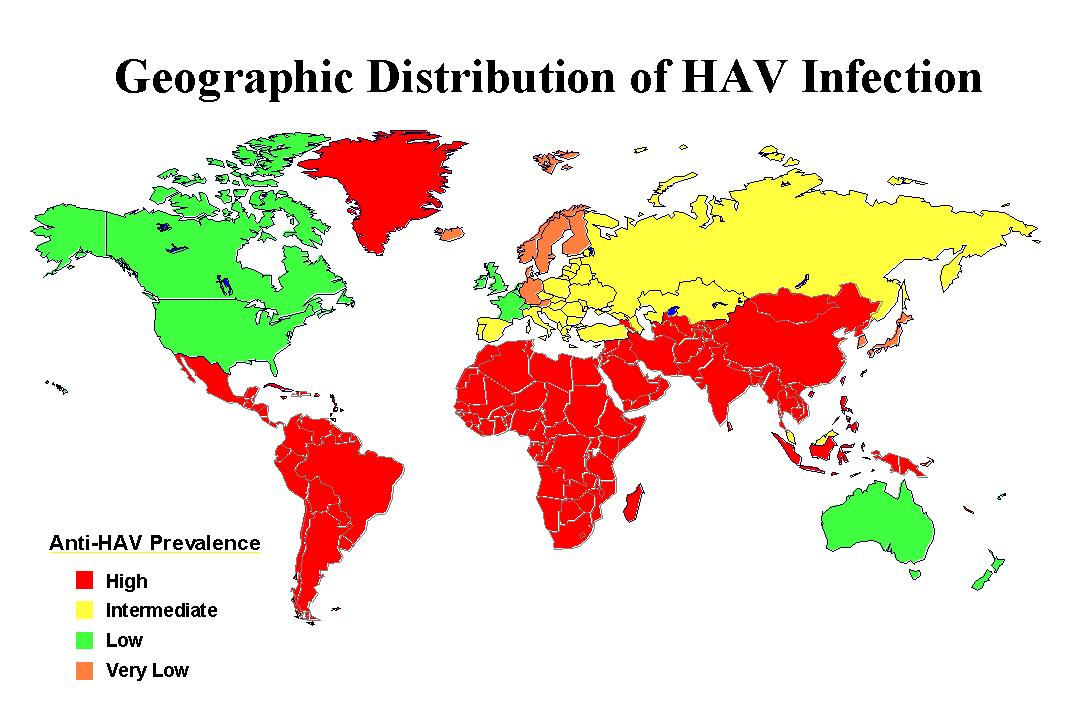
- Hepatitis A occurs throughout the world.
- It is highly endemic in some areas, particularly Central and South America, Africa, the Middle East, Asia, and the Western Pacific.
Transmission of Hepatitis A Virus
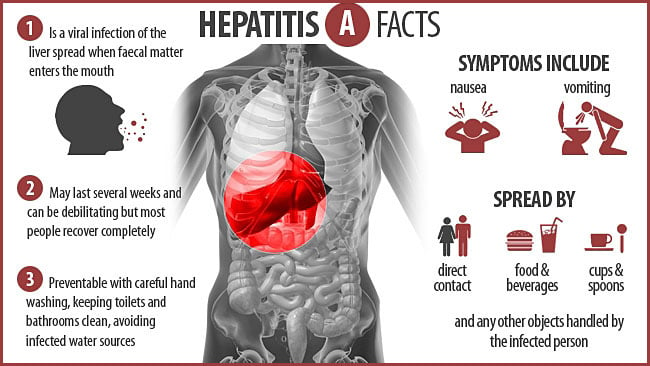
- Transmission via fecal-oral route; ingestion of fecally contaminated food (eating uncooked shellfish harvested from sewage) or contaminated water.
Replication of Hepatitis A Virus
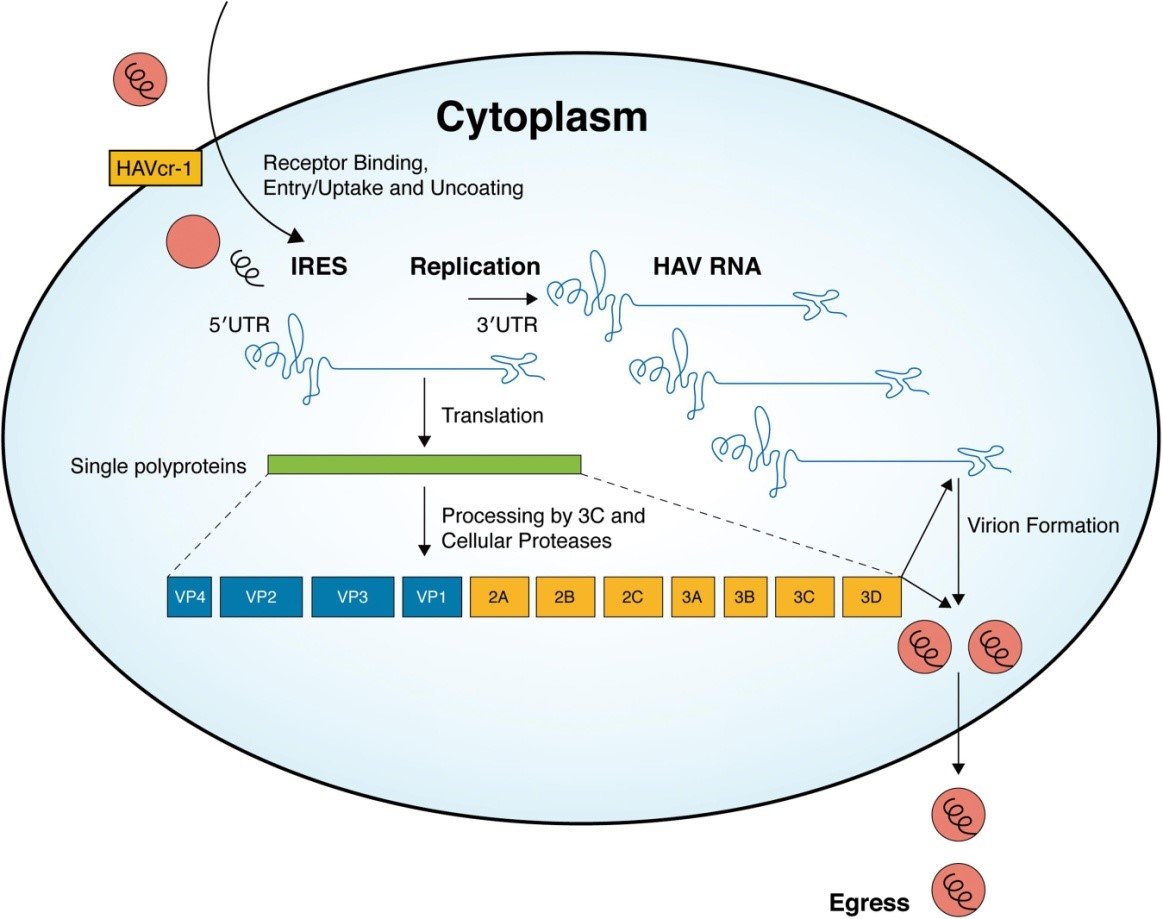
- HAV is spread primarily through the ingestion of fecally contaminated food or water.
- Once HAV reaches the intestine, it is thought to be absorbed into the bloodstream and to reach the liver through the portal system.
- Attachment of the virus to host cell receptors (HAV cr-1) mediates endocytosis of the virus into the host cell possibly by clathrin- dependent endocytosis.
- Upon endosomal acidification, the capsid undergoes a conformational change and release VP4 that opens a pore in the host endosomal membrane and the viral genomic RNA penetrates into the host cell cytoplasm.
- VPg protein is removed from the viral RNA, which is translated into a processed polyproten.
- The IRES allows direct translation of the polyprotein.
- A ds RNA genome is synthesized from the genomic ssRNA(+).
- The dsRNA genome is transcribed thereby providing viral mRNAs/new ssRNA(+) genomes.
- New genomic RNA is believed to be packaged into preassembled procapsids.
- Cell lysis occurs and virus is released.
Pathogenesis of Hepatitis A Virus
- Viral replication occurs primarily within hepatocytes and the secretion of virus into bile results in large quantities of virus being shed in the faeces.
- During the incubation period, viremia is observed at about the same time that fecal shedding of HAV is occurring.
- Viremia terminates shortly after hepatitis develops, whereas feces may remain infectious for another 1 to 2 weeks.
- Acute hepatitis includes features like inflammatory cell infiltration, hepatocellualr necrosis and liver cell regeneration.
- Portal infiltration by lymphocytes, plasma cells and periodic acid Schiff (PAS)-positive macrophages are prominent features in early biopsies.
- Parenchymal cells undergo ballooning degeneration.
- These hepatocytes are swollen and have indistinct plasma membranes, enlarged nuclei, and a featureless cytoplasm, except for some cytoplasmic remnants condensed around the nuclei.
- Disruption of bile canaliculi may lead to bile retention after liver cell enlargement or necrosis.
- In some cases, extension of the inflammatory infiltrate from the periportal region into the hepatic parenchyma with significant erosion of the limiting plate is seen.
- HAV replication in the liver triggers a substantial immune response, both humoral and cell mediated.
- CD8 +, cytotoxic T cells that are capable of lysing autologous HAV infected cells , but not of controlling uninfected cells, are present both in circulation and in the liver at the site of disease.
- These virus specific T cells also produce interferon gamma and other cytokines at the site of infection that may be responsible for much of liver injury.
- In addition to cell mediated immune response, there is vigorous antibody response to the virus during later stages of infection which are directed against conformational epitopes.
- Neutralizing antiviral antibodies play an important role in clearance of the virus.
- Serum antibody responses are first noted at onset of symptoms and include virus specific IgM as well as IgG and IgA.
Clinical Manifestations of Hepatitis A Virus
- HAV causes an acute, self limiting infection that does not progress to chronic phase.
- Following exposure, an incubation period of 15-45 days precedes the development of clinical symptoms.
- Virus is present in blood and shed in stools within a few days of exposure.
- Symptoms typically occurs abruptly, with liver injury heralded by fever, myalgia, nausea, anorexia and vomiting accompanied by right upper quadrant abdominal pain.
- Disruption of hepatobilary metabolism results in passage of dark coca cola like urine, light gray coloured stool and frank icterus.
- Appearance of biochemical abnormalities including abnormal elevation of liver derived enzymes like ALT- alanine aminotransferase, ALP- alkaline phosphatase, GGTP- gamma glutamyl transpeptidase and increased level of serum bilirubin.
- The severity of infection is associated with age of patient indicating individuals over 50 years of age at increased risk.
- They may developed fulminant hepatitis with clinical pictures including ascites, bleeding diathesis or hepatic coma.
- Severe complication includes cholestatic hepatitis characterized by persistent jaundice associated with pruritis, anorexia and weight loss.
Lab Diagnosis of Hepatitis A Virus
Sample: blood, stool, bile, liver biopsy, serum
- Antigen detection
- Detection using PCR and nucleic acid hybridization assay.
- Antibody detection
- Demonstration of IgM antibodies to the virus, which are almost always present at the onset of symptoms and which persist for up to 6 months following infection.
- IgG antibody usually persists for many years and is a useful indicator of immunity.
- Antibody detection done by Enzyme linked immunsorbent assay (ELISA).
- Liver function test- detection of level of liver enzymes like ALT, ALP, GGTP, and serum bilirubin.
Treatment of Hepatitis A Virus
- Supportive treatment to reduce other non specific symptoms
- No antiviral therapy
Vaccination of Hepatitis A Virus
- Inactivate or live attenuated vaccine confers 90% of prevention.
- Formalin inactivated HAV vaccine given in two doses- an initial dose followed by booster dose after 6-12 months.
- Two inactivated whole-virus hepatitis A vaccines are available: HAVRIX (GlaxoSmithKline) and VAQTA (Merck).
Prevention and control of Hepatitis A Virus
- Persons exposed to HAV (e.g., those who have been served food by an HAV-infected food handler) can be offered administration of serum immune globulin.
- If given soon after HAV exposure, the anti-HAV antibodies in serum immune globulin can prevent HAV infection or reduce its extent and severity.
- This form of preventive intervention is known as postexposure prophylaxis.
- Personnel hygiene and sanitation should be followed.

its very informative thank you so much for sharing such clear explanation .