Interesting Science Videos
Heat coagulation test of proteins definition
Heat coagulation test of protein is a biochemical test performed to determine the presence of proteins like albumin and globulin in protein. Coagulation of proteins as a response to heat is a common phenomenon. The heat coagulation of proteins occurs in one of the two stages; denaturation and agglutination or the separation of the denatured protein in a particular form. The heating of coagulable proteins at their isoelectric pH, a series of changes occur to the proteins; dissociation of subunits or the quaternary structure, uncoiling of the polypeptide chains ultimately leading to the matting together of uncoiled polypeptide chains. Processes like coagulation and flocculation are superficial visible manifestations of changes that occur in proteins during the denaturation process.
Coagulation of proteins is an irreversible process that is maximum at the isoelectric pH of the proteins. Heat coagulation of proteins is an important clinical test for the detection of proteinuria. It is simple and less time-consuming. Both qualitative and quantitative estimation of proteins can be done with the heat coagulation method. The quantitative analysis of coagulation can be performed by measuring the coagulum formed on the test tube.
Objectives of Heat coagulation test of proteins
- To detect the presence of proteins in a given sample.
- To detect the presence of albumin, globulin, and other proteins present in a urine sample.
Principle of Heat coagulation test of proteins
The principle of heat coagulation test is the change in the structure of proteins as a result of heat and change in pH. Heating a protein in acidic medium results in denaturation of protein due to the breaking of certain bonds responsible for the tertiary and quaternary structure of proteins. However, in the case of coagulable proteins, when these proteins are heated at their isoelectric pH, the polypeptide chains become uncoiled and matt with each other to form an insoluble mass. The mass formed doesn’t dissolve back to the liquid. The process of coagulation is maximum at the isoelectric point, and the mass of the coagulum might differ with the particle size and the concentration of proteins in the sample. For the heat coagulation test of albumin and globulin, chlorophenol red is used which adjusts the pH of the sample to the isoelectric point of albumin. The reagent for this test also contains acetic acid, which helps in the breaking of peptide bonds present in the protein molecule, facilitating coagulation.
Requirements
Reagent
- Chlorophenol red indicator
- 1% acetic acid
- Sample
Materials required
- Test tubes
- Test tube stand
- Pipettes
Procedure of Heat coagulation test of proteins
- About two-thirds of the test tube is filled with the given sample.
- To the test tube, 1-2 drops of chlorophenol red indicator is added drop by drop and mixed properly.
- When a purple color is observed on the test tube, 1% acetic acid is added drop by drop until the color changes to pale pink.
- The test tube is then inclined slightly so as to heat the upper portion of the fluid.
- The tube is observed for the formation of the coagulum.
Result and Interpretation of Heat coagulation test of proteins
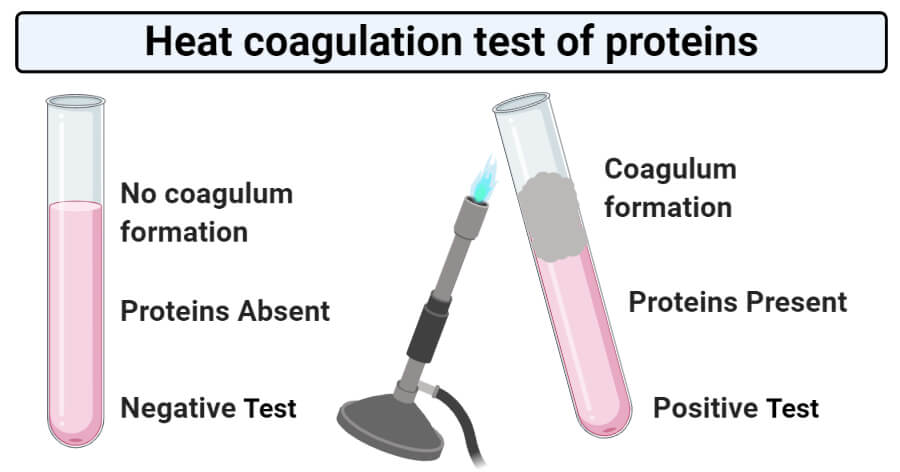
- Positive result: A positive result of the heat coagulation test is represented by the formation of a dense coagulum at the upper part of the solution. The lower part of the solution acts as a control.
- Negative result: A negative result of the heat coagulation test is represented by the absence of coagulum at the upper layer. This indicates the absence of albumin and other proteins in urine.
Uses of Heat coagulation test of proteins
- Heat coagulation test is used to detect the presence of albumin and globulin protein in the urine sample. As albumin and globulin in urine are observed under many pathological conditions, their presence can be conclusively established by this test, which aids in the diagnosis of diseases.
- The test is one of the most commonly used methods for the detection of proteins in the urine.
Limitations
- In some cases, the test might give a positive result for other coagulable proteins that might be present in the urine.
- It is imperative that the pH of the solution should be around the isoelectric pH of the proteins suspected to be in the sample.
References
- Lepeschkin, W W. “The Heat-Coagulation of Proteins.” The Biochemical journal 16,5 (1922): 678-701. doi:10.1042/bj0160678
- Mastin, H, and H G Rees. “The Heat Coagulation of Egg-Albumin.” The Biochemical journal 20. 4 (1926): 759-62. doi:10.1042/bj0200759
- Nigam S. C. and Omkar (2003). Experimental Animal Physiology and Biochemistry. New Age International Pvt. Limited. New Delhi.
- D (2012). Biochemistry. Fourteenth Edition. Academic Publishers. Kolkata.
- http://bioimdc.byethost24.com/wp-content/uploads/2014/03/17.-HEAT-COAGULATION-TEST.pptx
